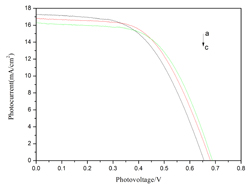
应用光谱电化学方法测定了纳米晶TiO2电极在不同浓度的4-叔丁基吡啶(TBP)电解液中的平带电势(Efb). TBP对纳米晶TiO2电极的能带结构具有显著的影响. 在不含和含有0.2或0.4 mol•L-1TBP的0.2 mol•L-1高氯酸四丁基铵(TBAP)/乙腈溶液中,测得TiO2电极的Efb依次为-2.25,-2.46和-2.60 V. 当加入Li+后,TiO2电极的Efb正移,在不含和含有0.2或0.4 mol•L-1TBP的0.2 mol•L-1 LiClO4/乙腈溶液中,测得TiO2电极的Efb依次为-1.12,-1.22和-1.30 V. 用时间分辨电流方法测定了陷阱态分布. 在不含和含有0.2或0.4 mol•L-1TBP的0.2 mol•L-1 TBAP/乙腈溶液中,TiO2电极的陷阱态密度依次为3.52 × 1016, 3.18 × 1016和3.37 × 1016 cm-2,陷阱态分布的最大值位于-1.99, -1.89和-1.85 V处. Li+的加入进一步减少了陷阱态密度. 在不含和含有0.2或0.4 mol•L-1TBP的0.2 mol•L-1 LiClO4/乙腈溶液中,TiO2电极的陷阱态密度依次为8.39 × 1015, 1.11 × 1016和9.22 × 1015 cm-2,陷阱态分布的最大值位于-0.72, -0.84和-0.95V处. 最后,研究了N3染料敏化的纳米晶TiO2电极在含有不同浓度TBP的电解质溶液中的光电化学性质. 结果显示,随着TBP浓度的增加,Voc增大,使TiO2电极的光电转化效率增加.
杨术明
,
王纪超
,
寇慧芝
,
薛洪滨
,
王红军
,
郭玉玲
. 4-叔丁基吡啶对纳米晶TiO2电极的能带结构及光电化学性质的影响[J]. 电化学, 2011
, 17(2)
: 204
-211
.
DOI: 10.61558/2993-074X.2090
The flat band edges (Efb) of nanostructured TiO2 electrodes in electrolyte solutions with tert-butylpyridine (TBP) of different concentrations have been determined with spectroelectrochemical technique. TBP played a role in band energetics of nanostructured TiO2 electrodes. The Efb values of -2.25, -2.46 and -2.62 V were determined in three 0.2 mol•L-1 tetrabutylammonium perchlorate (TBAP) acetonitrile electrolytes which contain 0, 0.2 and 0.4 mol•L-1 TBP respectively. The addition of Li+ ions shifted Efb positively. The Efb values of -1.12, -1.22 and -1.30 V were determined in three 0.2 mol•L-1 LiClO4 acetonitrile electrolytes which contain 0, 0.2 and 0.4 mol•L-1 TBP respectively. The trap state distribution was investigated by the measurements of time resolved current. The total trap state densities of 3.52 × 1016, 3.18 × 1016 and 3.37 × 1016 cm-2 were determined in three 0.2 mol•L-1 TBAP acetonitrile electrolytes which contain 0, 0.2 and 0.4 mol•L-1 TBP respectively with trap distribution maximum located at -1.99, -1.89 and -1.85 V. The addition of Li+ ions further reduced the trap state densities. The total trap state densities of 8.39 × 1015, 1.11 × 1016 and 9.22 × 1015 cm-2 were determined in three 0.2 mol•L-1 LiClO4 acetonitrile electrolytes which contain 0, 0.2 and 0.4 mol•L-1 TBP respectively with trap distribution maximum located at -0.72, -0.84 and -0.95 V. Finally the nanostructured TiO2 electrodes were sensitized with dye N3 and their photoelectrochemical properties were studied in electrolytes with TBP of different concentrations. Experiment results showed that as the concentration of TBP increased, the photoelectric conversion efficiency increased due to improved Voc.