

KOH和K3[Fe(CN) 6]混合液中颗粒孔径对NiO电极电容器性能的影响
收稿日期: 2010-12-02
修回日期: 2011-01-28
网络出版日期: 2011-05-06
基金资助
国家自然科学基金重点项目(20633040)和上海市科委(08DZ2270500)
Effect of Pore Size on Pseudo-Capacitive Performance of NiO in the Mixed Electrolyte of KOH and Hexacyanoferrate
Received date: 2010-12-02
Revised date: 2011-01-28
Online published: 2011-05-06
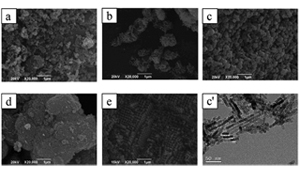
关键词: 超级电容器; NiO; 多孔材料; K3[Fe(CN) 6]
吴雯 , 侯孟炎 , 周丹丹 , 夏永姚 . KOH和K3[Fe(CN) 6]混合液中颗粒孔径对NiO电极电容器性能的影响[J]. 电化学, 2011 , 17(2) : 169 -174 . DOI: 10.61558/2993-074X.2085

Key words: supercapacitors; nickel oxides; porous materials; hexacyanoferrate
/
| 〈 |
|
〉 |