

片层纺锤形纳米CuO的制备及性能研究
收稿日期: 2011-03-08
修回日期: 2011-03-28
网络出版日期: 2011-05-06
基金资助
国家自然科学基金(21073018)
Synthesis and Catalytic Property of Flaked Spindle-like CuO Nanocrystals
Received date: 2011-03-08
Revised date: 2011-03-28
Online published: 2011-05-06
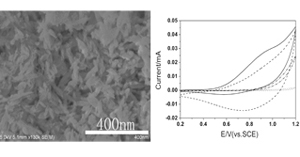
关键词: 片层纺锤形纳米CuO; 电化学; H2O2; 催化氧化
范建凤 , 李丽青 , 黄玉峰 , 范楼珍 . 片层纺锤形纳米CuO的制备及性能研究[J]. 电化学, 2011 , 17(2) : 186 -189 . DOI: 10.61558/2993-074X.2087

/
| 〈 |
|
〉 |