

模拟体液中镁合金表面PCL-TiO2@Ag/γ-CD MOF纳米复合涂层的耐腐蚀、生物相容和抗菌性研究
收稿日期: 2025-04-24
修回日期: 2025-06-24
录用日期: 2025-07-21
网络出版日期: 2025-07-21
Biocompatible and Antibacterial PCL-TiO2@Ag/γ-CD MOF Nanocomposite Coating for Corrosion Resistance of Magnesium Alloy in Simulated Body Fluid
Received date: 2025-04-24
Revised date: 2025-06-24
Accepted date: 2025-07-21
Online published: 2025-07-21
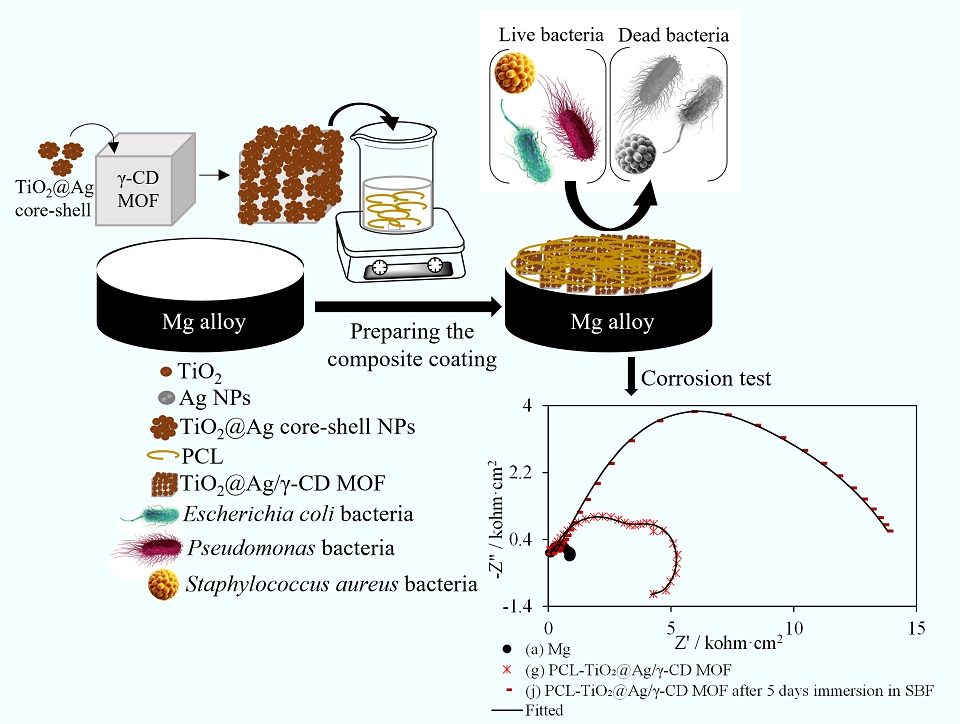
镁合金由于具有可降解性和生物相容性,被认为是生物植入物应用的有前景的候选材料。然而,其快速腐蚀仍然是实际应用的一个关键限制因素。本研究开发了一种多功能纳米复合涂层,旨在提高镁合金植入物的耐腐蚀性和抗菌性能。该涂层由表面修饰TiO2@Ag核-壳纳米颗粒的γ-环糊精金属有机框架(γ-CD MOF)构成,并嵌入到聚己内酯(PCL)基体中(PCL-TiO2@Ag/γ-CD MOF),与未修饰TiO2@Ag核-壳纳米颗粒的涂层(PCL/γ-CD MOF)进行比较。模拟体液浸泡测试结果表明,虽然PCL-TiO2@Ag/γ-CD MOF复合涂层初始的腐蚀速率高于PCL/γ-CD MOF涂层,但随着浸泡时间的推移,其性能显著改善。五天后,腐蚀抑制率达到95.44%,腐蚀速率降至1.70 mpy。此外,该复合涂层对大肠杆菌、假单胞菌和金黄色葡萄球菌均表现出较强的抗菌活性。研究证实,该涂层促进了成骨样MC3T3-E1细胞的生长和繁殖,从而具有无毒性和良好的生物相容性。本研究结果表明,PCL-TiO2@Ag/γ-CD MOF纳米复合涂层在可降解镁合金植入物中具有良好的生物相容性、抗菌性和耐腐蚀性,在生物医学领域具有广阔的应用前景。
关键词: 抗菌涂层; 生物相容性; 防腐蚀涂层; γ-环糊精金属有机框架; TiO2@Ag核-壳结构
萨拉·德赫甘-切纳尔 , 哈米德·礼萨·扎雷 , 扎赫拉·穆罕默德普尔 , 玛丽亚姆·萨达特·米尔巴盖里-菲鲁扎巴德 . 模拟体液中镁合金表面PCL-TiO2@Ag/γ-CD MOF纳米复合涂层的耐腐蚀、生物相容和抗菌性研究[J]. 电化学, 2025 , 31(11) : 2504241 . DOI: 10.61558/2993-074X.3573
Magnesium alloys are promising candidates for bio-implant applications due to their biodegradability and biocompatibility. However, their rapid corrosion remains a critical limitation. This study presents the development of a multifunctional nanocomposite coating designed to enhance the corrosion resistance and antibacterial properties of magnesium alloy implants. The coating comprised γ-cyclodextrin metal-organic frameworks (γ-CD MOFs) decorated with TiO2@Ag core-shell nanoparticles, embedded in a polycaprolactone (PCL) matrix. Immersion tests in a simulated body fluid (SBF) revealed an initially higher corrosion rate for the PCL-TiO2@Ag/γ-CD MOF coating compared to the coating without TiO2@Ag nanoparticles; however, it demonstrated significant improvement over time. After five days, the corrosion inhibition reached 95.44%, with the corrosion rate decreasing to 1.70 mpy. Additionally, the composite coating exhibited strong antibacterial activity against Escherichia coli, Pseudomonas, and Staphylococcus aureus. Furthermore, MTT assays indicated that the coating facilitated the growth and proliferation of osteoblast-like MC3T3-E1 cells, confirming its nontoxicity and biocompatibility. These findings highlight the potential of the PCL-TiO2@Ag/γ-CD MOF nanocomposite as a biocompatible, antibacterial, and corrosion-resistant coating for biodegradable magnesium implants, offering a promising solution for biomedical applications.

| [1] | Zhang T, Wang W, Liu J, Wang L Q, Tang Y J, Wang K S. A review on magnesium alloys for biomedical applications[J]. Front. bioeng. biotechnol., 2022, 10: 953344. https://doi.org/10.3389/fbioe.2022.953344. |
| [2] | He M F, Chen L X, Yin M, Xu S X, Liang Z Y. Review on magnesium and magnesium-based alloys as biomaterials for bone immobilization[J]. J. Mater. Res. Technol., 2023, 23: 4396-4419. https://doi.org/10.1016/j.jmrt.2023.02.037. |
| [3] | Tsakiris V, Tardei C, Clicinschi F. M. Biodegradable Mg alloys for orthopedic implants-A review[J]. J. Magnes. Alloy, 2021, 9(6): 1884-1905. https://doi.org/10.1016/j.jma.2021.06.024. |
| [4] | Peng F, Li H, Wang D H, Tian P, Tian Y Z, Yuan G Y, Xu D M, Liu X Y. Enhanced corrosion resistance and biocompatibility of magnesium alloy by Mg-Al-layered double hydroxide[J]. ACS Appl. Mater. Interfaces, 2016, 8(51): 35033-35044. https://doi.org/10.1021/acsami.6b12974. |
| [5] | Xu L P, Yu G N, Zhang E, Pan F, Yang K. In vivo corrosion behavior of Mg‐Mn‐Zn alloy for bone implant application[J]. J. Biomed. Mater. Res. A. 2007, 83(3): 703-711. https://doi.org/10.1002/jbm.a.31273. |
| [6] | Abdel Aal A. Protective coating for magnesium alloy[J]. J. Mater. Sci., 2008, 43: 2947-2954. https://doi.org/10.1007/s10853-007-1796-2. |
| [7] | Yang J X, Cui F Z, Lee I. S. Surface modifications of magnesium alloys for biomedical applications[J]. Ann. Biomed. Eng., 2011, 39: 1857-1871. https://doi.org/10.1007/s10439-011-0300-y. |
| [8] | Zhang G, Wu L, Tang A T, Ma Y L, Song G L, Zheng D J, Jiang B, Atrens A, Pan F. Active corrosion protection by a smart coating based on a MgAl-layered double hydroxide on a cerium-modified plasma electrolytic oxidation coating on Mg alloy AZ31[J]. Corros. Sci., 2018, 139: 370-382. https://doi.org/10.1016/j.corsci.2018.05.010. |
| [9] | Ren Y, Babaie E, Bhaduri S. B. Nanostructured amorphous magnesium phosphate/poly (lactic acid) composite coating for enhanced corrosion resistance and bioactivity of biodegradable AZ31 magnesium alloy[J]. Prog. Org. Coat., 2018, 118: 1-8. https://doi.org/10.1016/j.porgcoat.2018.01.014. |
| [10] | Johnson I, Akari K, Liu H. Nanostructured hydroxyapatite/poly (lactic-co-glycolic acid) composite coating for controlling magnesium degradation in simulated body fluid[J]. Nanotechnol., 2013, 24(37): 375103. https://doi.org/10.1088/0957-4484/24/37/375103. |
| [11] | Carangelo A, Acquesta A, Monetta T. In-vitro corrosion of AZ31 magnesium alloys by using a polydopamine coating[J]. Bioact. Mater., 2019, 4: 71-78. https://doi.org/10.1016/j.bioactmat.2018.12.005. |
| [12] | Shah S. A, Sohail M, Nakielski P, Rinoldi C, Zargarian S S, Kosik-Kozioł A, Ziai Y, Haghighat Bayan M A, Zakrzewska A, Rybak D. Integrating micro-and nanostructured platforms and biological drugs to enhance biomaterial-based bone regeneration strategies[J]. Biomacromolecules, 2024, 26(1): 140-162. https://doi.org/10.1021/acs.biomac.4c01133. |
| [13] | Mena-Morcillo E, Veleva L, Cerda-Zorrilla M, Soria-Castro M, Castro-Alcántara J C, Canul-Puc R C. Development and assessment of a multifunctional chitosan-based coating applied on AZ31 magnesium alloy: Corrosion resistance and antibacterial performance against Klebsiella Pneumoniae[J]. J. Magnes. Alloy, 2021, 9(6): 2133-2144. https://doi.org/10.1016/j.jma.2021.03.033. |
| [14] | Xu W, Yagoshi K, Asakura T, Sasaki M, Niidome T. Silk fibroin as a coating polymer for sirolimus-eluting magnesium alloy stents[J]. ACS Appl. Bio Mater., 2019, 3(1): 531-538. https://doi.org/10.1021/acsabm.9b00957. |
| [15] | Malikmammadov E, Tanir T. E, Kiziltay A, Hasirci V, Hasirci N. PCL and PCL-based materials in biomedical applications[J]. J. Biomater. Sci. Polym. Ed., 2018, 29(7-9): 863-893. https://doi.org/10.1080/09205063.2017.1394711. |
| [16] | Wong H. M, Yeung K. W, Lam K. O, Tam V, Chu P. K, Luk K. D, Cheung K. M. A biodegradable polymer-based coating to control the performance of magnesium alloy orthopaedic implants[J]. Biomater. 2010, 31(8): 2084-2096. https://doi.org/10.1016/j.biomaterials.2009.11.111. |
| [17] | Kim H K, Jang S J, Cho Y S, Park H H. Fabrication of nanostructured polycaprolactone (PCL) film using a thermal imprinting technique and assessment of antibacterial function for its application[J]. Polymers, 2022, 14(24): 5527. https://doi.org/10.3390/polym14245527. |
| [18] | Gao Y, Hassanbhai A. M, Lim J, Wang L, Xu C. Fabrication of a silver octahedral nanoparticle-containing polycaprolactone nanocomposite for antibacterial bone scaffolds[J]. RSC Adv., 2017, 7(17): 10051-10056. https://doi.org/10.1039/C6RA26063B. |
| [19] | Singh N, Batra U, Kumar K, Mahapatro A. Evaluation of corrosion resistance, mechanical integrity loss and biocompatibility of PCL/HA/TiO2 hybrid coated biodegradable ZM21 Mg alloy[J]. J. Magnes. Alloy, 2022, 10 (11): 3179-3204. https://doi.org/10.1016/j.jma.2021.10.004. |
| [20] | Rojas S, Devic T, Horcajada P. Metal organic frameworks based on bioactive components[J]. J. Mater. Chem. B., 2017, 5(14): 2560-2573. https://doi.org/10.1039/C6TB03217F. |
| [21] | Han Y Y, Liu W C, Huang J J, Qiu S W, Zhong H R, Liu D, Liu J Q. Cyclodextrin-based metal-organic frameworks (CD-MOFs) in pharmaceutics and biomedicine[J]. Pharm., 2018, 10(4): 271. https://doi.org/10.3390/pharmaceutics10040271. |
| [22] | Patyk-Kaźmierczak E, Warren M. R, Allan D. R, Katrusiak A. Pressure inverse solubility and polymorphism of an edible γ-cyclodextrin-based metal-organic framework[J]. Phys. Chem. Chem. Phys., 2017, 19(13): 9086-9091. https://doi.org/10.1039/C7CP00593H. |
| [23] | Trushina D B, Sapach A Y, Burachevskaia O A, Medvedev P V, Khmelenin D N, Borodina T. N, Soldatov M A, Butova V V. Doxorubicin-loaded core-shell UiO-66@SiO2 metal-organic frameworks for targeted cellular uptake and cancer treatment[J]. Pharm., 2022, 14(7): 1325. https://doi.org/10.3390/pharmaceutics14071325. |
| [24] | Abd El-Fattah W, Alfaifi M Y, Alkabli J, Ramadan H A, Shati A A, Elbehairi S E I, Elshaarawy R F, Kamal I, Saleh M M. Immobilization of ZnO-TiO2 nanocomposite into polyimidazolium amphiphilic chitosan film targeting improving its antimicrobial and antibiofilm applications[J]. Antibiotics, 2023, 12(7): 1110. https://doi.org/10.3390/antibiotics12071110. |
| [25] | Phan D N, Dorjjugder N, Saito Y, Taguchi G, Lee H, Lee J S, Kim I S. The mechanistic actions of different silver species at the surfaces of polyacrylonitrile nanofibers regarding antibacterial activities[J]. Mater. Today Commun., 2019, 21: 100622. https://doi.org/10.1016/j.mtcomm.2019.100622. |
| [26] | Ji X H, Ji W H, Pourhashem S, Wang W, Duan J Z, Hou B. (TiO2/Ag nanoparticles)@(chitosan-polyvinyl alcohol) core-shell nanofibers as novel anticorrosion and antibacterial improvers for epoxy coating systems[J]. J. Polym. Res., 2023, 30(6): 244. https://doi.org/10.1007/s10965-023-03569-x. |
| [27] | Nakata K, Fujishima A. TiO2 photocatalysis: Design and applications[J]. J. Photochem. Photobiol. C: Photochem. Rev., 2012, 13(3): 169-189. https://doi.org/10.1016/j.jphotochemrev.2012.06.001. |
| [28] | Dehghan-Chenar S, Zare H R, Mohammadpour Z. Chitosan-ImH@γ-CD: a pH-sensitive smart bio-coating to enhance the corrosion resistance of magnesium alloys in bio-implants[J]. RSC adv., 2024, 14 (45): 33301-33310. https://doi.org/10.1039/D4RA04744C. |
| [29] | Rocha M, Pereira C, Freire C. Au/Ag nanoparticles-decorated TiO2 with enhanced catalytic activity for nitroarenes reduction[J]. Colloids Surf. Physicochem. Eng. Aspects., 2021, 621: 126614. https://doi.org/10.1016/j.colsurfa.2021.126614. |
| [30] | Wang Z J, Ma Y D, Jiang Y, Zhou F, Wu Y L, Jiang H T, Wang R L, Xu Q, Hua C. Encapsulating quercetin in cyclodextrin metal-organic frameworks improved its solubility and bioavailability[J]. J. Sci. Food Agric., 2022, 102(9): 3887-3896. https://doi.org/10.1002/jsfa.11738. |
| [31] | Ganapathy M, Senthilkumar N, Vimalan M, Jeysekaran R, Potheher I. V. Studies on optical and electrical properties of green synthesized TiO2@Ag core-shell nanocomposite material[J]. Mater. Res. Express., 2018, 5(4): 045020. https://doi.org/10.1088/2053-1591/aab91b. |
| [32] | Parvathiraja C, Shailajha S. Plasmonic core-shell nanoparticles of Ag@TiO2 for photocatalytic degradation of Rhodamine B[J]. Appl. Nanosci., 2023, 13(6): 3677-3692. https://doi.org/10.1007/s13204-022-02499-2. |
| [33] | Chen H P, Shan Y P, Xu C L, Bilal M, Zhao P Y, Cao C, Zhang H J, Huang Q L, Cao L D. Multifunctional γ-cyclodextrin-based metal-organic frameworks as avermectins carriers for controlled release and enhanced acaricidal activity[J]. ACS Agric. Sci. Technol., 2023, 3(2): 190-202. https://doi.org/10.1021/acsagscitech.2c00295. |
| [34] | Min Y Z, Song G, Zhou L, Wang X Y, Liu P Y, Li J M. Silver@mesoporous anatase TiO2 core-shell nanoparticles and their application in photocatalysis and SERS sensing[J]. Coatings, 2022, 12(1): 64. https://doi.org/10.3390/coatings12010064. |
| [35] | Mahajan J, Jeevanandam P. Novel thermal decomposition approach for the synthesis of TiO2@Ag core-shell nanocomposites and their application for catalytic reduction of 4-nitrophenol[J]. J. Nanopart. Res., 2019, 21: 1-17. https://doi.org/10.1007/s11051-019-4500-y. |
| [36] | Mohammadpour Z, Zare H R. Fabrication of a pH-sensitive epoxy nanocomposite coating based on a Zn-BTC metal-organic framework containing benzotriazole as a smart corrosion inhibitor[J]. Cryst. Growth Des., 2021, 21(7): 3954-3966. https://doi.org/10.1021/acs.cgd.1c00284. |
| [37] | Dehghan‐Chenar S, Zare H R, Mohammadpour Z. Fabrication of a curcumin@Cu‐BTC MOF smart anti‐corrosion coating for copper[J]. ChemistrySelect, 2023, 8(45): e202303392. https://doi.org/10.1002/slct.202303392. |
| [38] | Makkar P, Kang H J, Padalhin A R, Park I, Moon B G, Lee B T. Development and properties of duplex MgF2/PCL coatings on biodegradable magnesium alloy for biomedical applications[J]. PLoS One 2018, 13(4): e0193927. https://doi.org/10.1371/journal.pone.0193927. |
| [39] | Zheng Q Y, Li J, Yuan W, Liu X M, Tan L, Zheng Y F, Yeung K W K, Wu S. Metal-organic frameworks incorporated polycaprolactone film for enhanced corrosion resistance and biocompatibility of Mg alloy[J]. ACS Sustain. Chem. Eng., 2019, 7(21): 18114-18124. https://doi.org/10.1021/acssuschemeng.9b05196. |
| [40] | Jamesh M, Kumar S, Sankara Narayanan T. Electrodeposition of hydroxyapatite coating on magnesium for biomedical applications[J]. J. Coat. Technol. Res., 2012, 9: 495-502. https://doi.org/10.1007/s11998-011-9382-6. |
| [41] | Srinivasan A, Ranjani P, Rajendran N. Electrochemical polymerization of pyrrole over AZ31 Mg alloy for biomedical applications[J]. Electrochim. Acta, 2013, 88: 310-321. https://doi.org/10.1016/j.electacta.2012.10.087. |
| [42] | Fekry A, El-Sherif R. M. Electrochemical corrosion behavior of magnesium and titanium alloys in simulated body fluid[J]. Electrochim. Acta, 2009, 54(28): 7280-7285. https://doi.org/10.1016/j.electacta.2009.07.047. |
| [43] | Zhang S X, Li J N, Song Y, Zhao C L, Zhang X N, Xie C Y, Zhang Y, Tao H R, He Y H, Jiang Y J. In vitro degradation, hemolysis and MC3T3-E1 cell adhesion of biodegradable Mg-Zn alloy[J]. Mater. Sci. Eng. C, 2009, 29(6): 1907-1912. https://doi.org/10.1016/j.msec.2009.03.001. |
| [44] | Gonzalez J, Hou R Q, Nidadavolu E P, Willumeit-Römer R, Feyerabend F. Magnesium degradation under physiological conditions-Best practice[J]. Bioact. Mater., 2018, 3(2): 174-185. https://doi.org/10.1016/j.bioactmat.2018.01.003. |
| [45] | Skoog D A, West D M, Holler F J, Crouch S R. Fundamentals of analytical chemistry((9th ed.)[M]. Cengage Learning, 2013. |
| [46] | Zheng Y. F, Gu X. N, Witte F. Biodegradable metals[J]. Mater. Sci. Eng. R Rep., 2014, 77: 1-34. https://doi.org/10.1016/j.mser.2014.01.001. |
| [47] | Zamani Khalajabadi S, Haji Abu A B, Ahmad N, Kadir M R A, Ismail A F, Nasiri R, Haider W, Redzuan N B H. Biodegradable Mg/HA/TiO2 nanocomposites coated with MgO and Si/MgO for orthopedic applications: A study on the corrosion, surface characterization, and biocompatability[J]. Coatings, 2017, 7(10): 154. https://doi.org/10.3390/coatings7100154. |
| [48] | Song Y W, Shan D Y, Chen R S, Zhang F, Han E H. Biodegradable behaviors of AZ31 magnesium alloy in simulated body fluid[J]. Mater. Sci. Eng. C, 2009, 29(3): 1039-1045. https://doi.org/10.1016/j.msec.2008.08.026. |
| [49] | Shi Y, Qi M, Chen Y, Shi P. MAO-DCPD composite coating on Mg alloy for degradable implant applications[J]. Mater. Lett., 2011, 65(14): 2201-2204. https://doi.org/10.1016/j.matlet.2011.04.037. |
| [50] | Martin R, Brown P. The effects of magnesium on hydroxyapatite formation in vitro from CaHPO4 and Ca4(PO4)2O at 37.4 °C[J]. Calcif. Tissue Int., 1997, 60: 538-546. https://doi.org/10.1007/s002239900277. |
| [51] | Antoniac I, Adam R, Biță A, Miculescu M, Trante O, Petrescu I. M, Pogărășteanu M. Comparative assessment of in vitro and in vivo biodegradation of Mg-1Ca magnesium alloys for orthopedic applications[J]. Materials, 2020, 14(1): 84. https://doi.org/10.3390/ma14010084. |
/
| 〈 |
|
〉 |