

网络出版日期: 2025-05-16
Ionic Liquid Enhanced Proton Transfer for Neutral Oxygen Evolution Reaction
Online published: 2025-05-16
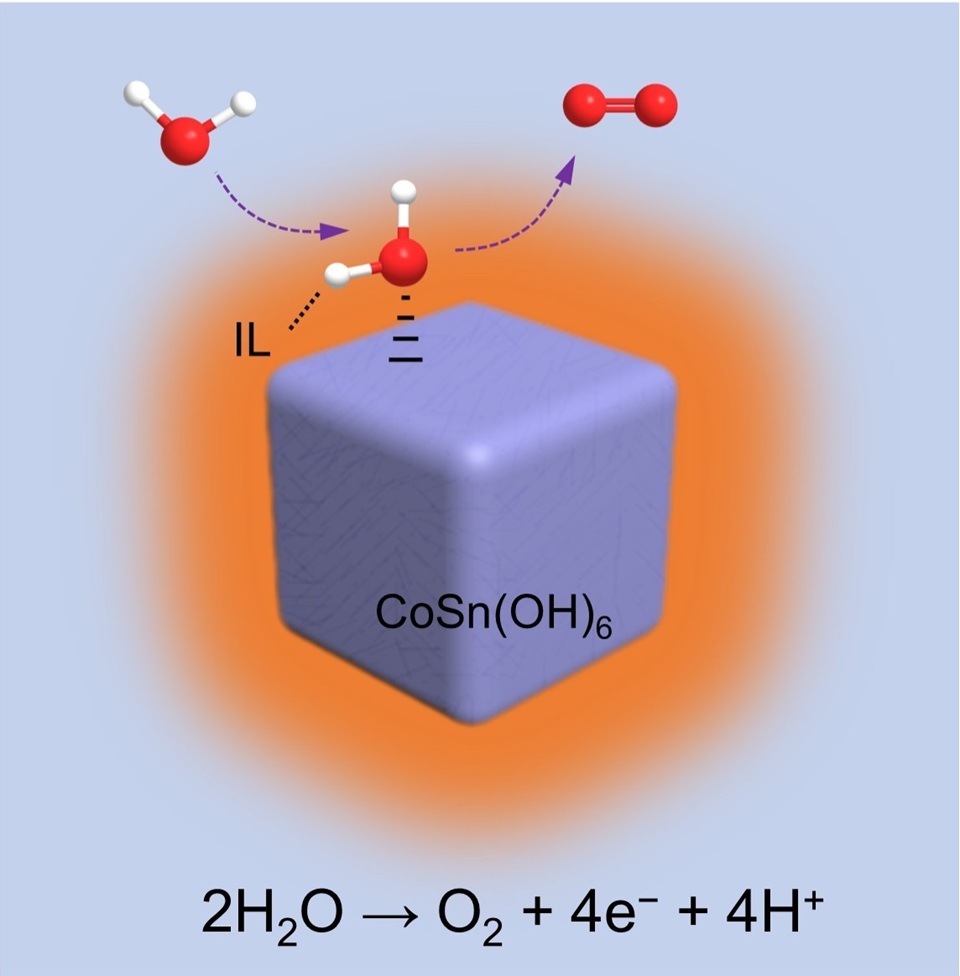
关键词: 电催化; 析氧反应; 离子液体; 质子转移; CoSn(OH)6立方体
陈明星, 刘念, 杜子翯, 齐静, 曹睿 . 离子液体增强质子转移并促进中性析氧反应[J]. 电化学, 0 : 0 . DOI: 10.61558/2993-074X.3549

/
| 〈 |
|
〉 |