

固态电解质反应器驱动的大气环境CO2捕集与电催化转化
收稿日期: 2025-04-04
修回日期: 2025-05-05
录用日期: 2025-05-13
网络出版日期: 2025-05-13
Ambient CO2 Capture and Valorization Enabled by Tandem Electrolysis Using Solid-State Electrolyte Reactor
Received date: 2025-04-04
Revised date: 2025-05-05
Accepted date: 2025-05-13
Online published: 2025-05-13
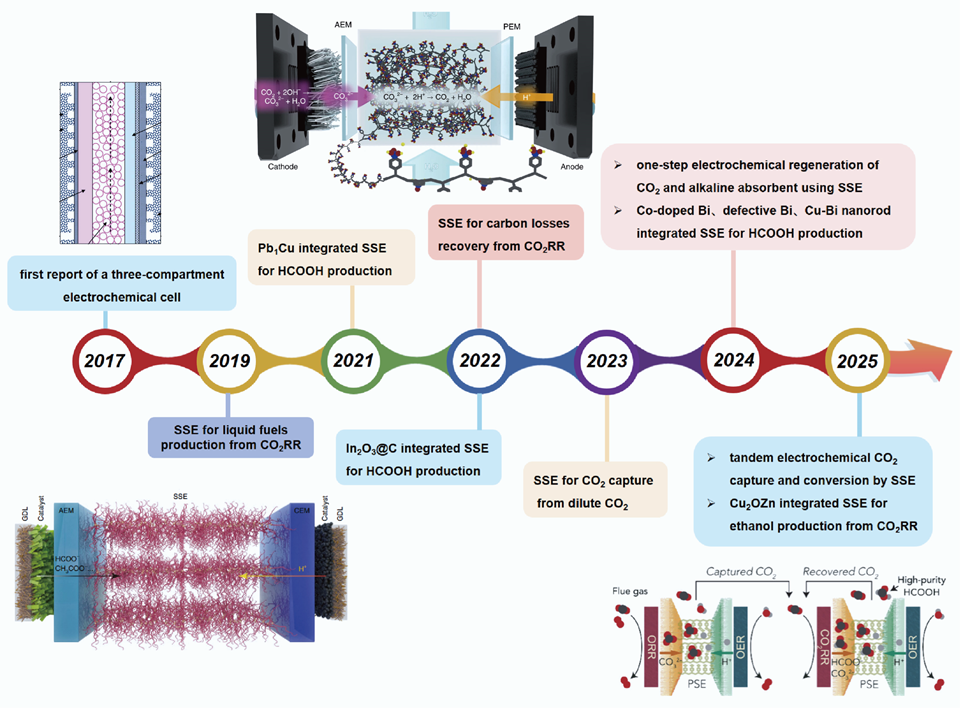
电催化二氧化碳还原是一种有望解决全球能源和环境危机的变革性技术。然而,其实际应用面临着两大关键挑战:一是分离混合还原产物的过程复杂且能耗高,二是所使用碳源(反应物)的经济可行性。为了同时解决这些挑战,固态电解质(SSE)反应器的研究正在受到日益广泛的关注。在这篇综述中,我们着眼于探讨将SSE应用于电化学CO2捕获和转化串联系统的可行性。我们首先讨论了SSE反应器的结构和基本原理,随后介绍了其在上述两个领域及串联电解的应用实例。与传统的H型电解池、流动池及膜电极电解池相比,SSE的关键创新在于阴离子交换膜和阳离子交换膜之间部署的SSE层,它实现了高效的离子传输,且可通过去离子水或湿润的氮气流有效地分离离子传导和产物收集功能。目标产物可以在SSE中间层通过两极离子复合形成,并通过多孔的SSE层被流动介质高效地带走,产生纯净的液相产物。由于CO2还原反应可以生成一系列液体产物,过去几年中先进催化剂的开发也进一步推动了SSE反应器在高效化学品生产中的实践应用。值得注意的是,由于阴极还原反应常常消耗水中的质子并导致局部高碱性环境,SSE可应用于从不同气源(如烟道气)中捕获酸性CO2以形成碳酸根离子。在电场的驱动下,形成的CO32-可以通过阴离子交换膜,并被阳极半反应产生的质子所酸化,实现高浓度CO2的再生,进而被收集作为下游CO2电还原的低成本原料。基于这一原理,近年来已有多种SSE构型的反应器被报道用于高效捕获不同气源的CO2。通过两个SSE单元的协同作用,已经实现了串联电化学CO2捕获和电催化转化。最后,我们对SSE在未来面向碳中和领域的应用中提出了展望,并建议更多关注以下具体方面的优化:SSE层的基本物理化学性质、电化学工程视角下离子和物种通量及选择性,以及连续CO2捕获和转化单元之间的系统性匹配。这些努力旨在进一步推动固态电解质反应器在更广泛的电化学领域中的应用示范。
华炎波 , 倪宝鑫 , 蒋昆 . 固态电解质反应器驱动的大气环境CO2捕集与电催化转化[J]. 电化学, 2025 , 31(6) : 2504082 . DOI: 10.61558/2993-074X.3547
Electrocatalytic carbon dioxide reduction is a promising technology for addressing global energy and environmental crises. However, its practical application faces two critical challenges: the complex and energy-intensive process of separating mixed reduction products and the economic viability of the carbon sources (reactants) used. To tackle these challenges simultaneously, solid-state electrolyte (SSE) reactors are emerging as a promising solution. In this review, we focus on the feasibility of applying SSE for tandem electrochemical CO2 capture and conversion. The configurations and fundamental principles of SSE reactors are first discussed, followed by an introduction to its applications in these two specific areas, along with case studies on the implementation of tandem electrolysis. In comparison to conventional H-type cell, flow cell and membrane electrode assembly cell reactors, SSE reactors incorporate gas diffusion electrodes and utilize a solid electrolyte layer positioned between an anion exchange membrane (AEM) and a cation exchange membrane (CEM). A key innovation of this design is the sandwiched SSE layer, which enhances efficient ion transport and facilitates continuous product extraction through a stream of deionized water or humidified nitrogen, effectively separating ion conduction from product collection. During electrolysis, driven by an electric field and concentration gradient, electrochemically generated ions (e.g., HCOO- and CH3COO-) migrate through the AEM into the SSE layer, while protons produced from water oxidation at the anode traverse the CEM into the central chamber to maintain charge balance. Targeted products like HCOOH can form in the middle layer through ionic recombination and are efficiently carried away by the flowing medium through the porous SSE layer, in the absence of electrolyte salt impurities. As CO2RR can generate a series of liquid products, advancements in catalyst discovery over the past several years have facilitated the industrial application of SSE for more efficient chemicals production. Also noteworthy, the cathode reduction reaction can readily consume protons from water, creating a highly alkaline local environment. SSE reactors are thereby employed to capture acidic CO2, forming CO32- from various gas sources including flue gases. Driven by the electric field, the formed CO32- can traverse through the AEM and react with protons originating from the anode, thereby regenerating CO2. This CO2 can then be collected and utilized as a low-cost feedstock for downstream CO2 electrolysis. Based on this principle, several cell configurations have been proposed to enhance CO2 capture from diverse gas sources. Through the collaboration of two SSE units, tandem electrochemical CO2 capture and conversion has been successfully implemented. Finally, we offer insights into the future development of SSE reactors for practical applications aimed at achieving carbon neutrality. We recommend that greater attention be focused on specific aspects, including the fundamental physicochemical properties of the SSE layer, the electrochemical engineering perspective related to ion and species fluxes and selectivity, and the systematic pairing of consecutive CO2 capture and conversion units. These efforts aim to further enhance the practical application of SSE reactors within the broader electrochemistry community.

| [1] | Seh Z W, Kibsgaard J, Dickens C F, Chorkendorff I, Norskov J K, Jaramillo T F. Combining theory and experiment in electrocatalysis: Insights into materials design[J]. Science, 2017, 355(6321): eaad4998. https://doi.org/10.1126/science.aad4998 |
| [2] | Liu F, Shi C X, Guo X L, He Z X, Pan L, Huang Z F, Zhang X W, Zou J J. Rational design of better hydrogen evolution electrocatalysts for water splitting: A review[J]. Adv. Sci., 2022, 9(18): 2200307.https://doi.org/10.1002/advs.202200307 |
| [3] | Wang L, Xu Z, Kuo C H, Peng J, Hu F, Li L, Chen H Y, Wang J, Peng S. Stabilizing low-valence single atoms by constructing metalloid tungsten carbide supports for efficient hydrogen oxidation and evolution[J]. Angew. Chem. Int. Ed., 2023, 62(42): e202311937. https://doi.org/10.1002/anie.202311937 |
| [4] | Chen J Y, Kang Y K, Zhang W, Zhang Z H, Chen Y, Yang Y, Duan L L, Li Y F, Li W. Lattice-confined single-atom Fe1Sx on mesoporous TiO2 for boosting ambient electrocatalytic N2 reduction reaction[J]. Angew. Chem. Int. Ed., 2022, 61(27): e202203022. https://doi.org/10.1002/anie.202203022 |
| [5] | Yang B, Ding W L, Zhang H H, Zhang S J. Recent progress in electrochemical synthesis of ammonia from nitrogen: Strategies to improve the catalytic activity and selectivity[J]. Energy Environ. Sci., 2021, 14(2): 672-687.https://doi.org/10.1039/d0ee02263b |
| [6] | Wang G X, Chen J X, Ding Y C, Cai P W, Yi L C, Li Y, Tu C Y, Hou Y, Wen Z H, Dai L M. Electrocatalysis for CO2 conversion: From fundamentals to value-added products[J]. Chem. Soc. Rev., 2021, 50(8): 4993-5061.https://doi.org/10.1039/d0cs00071j |
| [7] | Jiang M H, Wang H Z, Zhu M F, Luo X J, He Y, Wang M J, Wu C J, Zhang L Y, Li X M, Liao X, Jiang Z J, Jin Z. Review on strategies for improving the added value and expanding the scope of CO2 electroreduction products[J]. Chem. Soc. Rev., 2024, 53(10): 5149-5189.https://doi.org/10.1039/d3cs00857f |
| [8] | Pan F P, Fang L Z, Li B Y, Yang X X, O’carroll T, Li H Y, Li T, Wang G F, Chen K J, Wu G. N and OH-immobilized Cu3 clusters in situ reconstructed from single-metal sites for efficient CO2 electromethanation in bicontinuous mesochannels[J]. J. Am. Chem. Soc., 2024, 146(2): 1423-1434.https://doi.org/10.1021/jacs.3c10524 |
| [9] | Zhu N N, Zhang X Y, Chen N N, Zhu J H, Zheng X Y, Chen Z, Sheng T, Wu Z C, Xiong Y J. Integration of MnO2 nanosheets with Pd nanoparticles for efficient CO2 electroreduction to methanol in membrane electrode assembly electrolyzers[J]. J. Am. Chem. Soc., 2023, 145(45), 24852-24861.https://doi.org/10.1021/jacs.3c09307 |
| [10] | Xia W, Xie Y J, Jia S Q, Han S T, Qi R J, Chen T, Xing X Q, Yao T, Zhou D W, Dong X, Zhai J X, Li J J, He J P, Jiang D, Yamauchi Y, He M Y, Wu H H, Han B X. Adjacent copper single atoms promote C-C coupling in electrochemical CO2 reduction for the efficient conversion of ethanol[J]. J. Am. Chem. Soc., 2023, 145(31): 17253-17264.https://doi.org/10.1021/jacs.3c04612 |
| [11] | Kuhl K P, Cave E R, Abram D N, Jaramillo T F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces[J]. Energy Environ. Sci., 2012, 5(5): 7050.https://doi.org/10.1039/c2ee21234j |
| [12] | Jin S, Hao Z M, Zhang K, Yan Z H, Chen J. Advances and challenges for the electrochemical reduction of CO2 to CO: From fundamentals to industrialization[J]. Angew. Chem. Int. Ed., 2021, 60(38): 20627-20648.https://doi.org/10.1002/anie.202101818 |
| [13] | Nankya R, Elgazzar A, Zhu P, Chen F Y, Wang H T. Catalyst design and reactor engineering for electrochemical CO2 reduction to formate and formic acid[J]. Mater. Today, 2024, 76: 94-109.https://doi.org/10.1016/j.mattod.2024.05.002 |
| [14] | Lu T R, Xu T, Zhu S J, Li J, Wang J C, Jin H L, Wang X, Lv J J, Wang Z J, Wang S. Electrocatalytic CO2 reduction to ethylene: From advanced catalyst design to industrial applications[J]. Adv. Mater., 2023, 35(52): e2310433.https://doi.org/10.1002/adma.202310433 |
| [15] | Shin H, Hansen K U, Jiao F. Techno-economic assessment of low-temperature carbon dioxide electrolysis[J]. Nat. Sustain, 2021, 4(10): 911-919.https://doi.org/10.1038/s41893-021-00739-x |
| [16] | Gao W L, Liang S Y, Wang R J, Jiang Q, Zhang Y, Zheng Q W, Xie B Q, Toe C Y, Zhu X C, Wang J Y, Huang L, Gao Y S, Wang Z, Jo C, Wang Q, Wang L D, Liu Y F, Louis B, Scott J, Roger A C, Amal R, He H, Park S E. Industrial carbon dioxide capture and utilization: State of the art and future challenges[J]. Chem. Soc. Rev., 2020, 49(23): 8584-8686.https://doi.org/10.1039/D0CS00025F |
| [17] | Sullivan I, Goryachev A, Digdaya I A, Li X Q, Atwater H A, Vermaas D A, Xiang C X. Coupling electrochemical CO2 conversion with CO2 capture[J]. Nat. Catal., 2021, 4(11): 952-958.https://doi.org/10.1038/s41929-021-00699-7 |
| [18] | Lin L, Meng Y, Ju T Y, Han S Y, Meng F Z, Li J L, Du Y F, Song M Z, Lan T, Jiang J G. Characteristics, application and modeling of solid amine adsorbents for CO2 capture: A review[J]. J. Environ. Manage., 2023, 325(Pt A): 116438.https://doi.org/10.1016/j.jenvman.2022.116438 |
| [19] | Zhou Z H, Ma T Q, Zhang H Y, Chheda S, Li H Z, Wang K Y, Ehrling S, Giovine R, Li C S, Alawadhi A H, Abduljawad M M, Alawad M O, Gagliardi L, Sauer J, Yaghi O M. Carbon dioxide capture from open air using covalent organic frameworks[J]. Nature, 2024, 635(8037): 96-101.https://doi.org/10.1038/s41586-024-08080-x |
| [20] | Zhou Y, Zhang J L, Wang L, Cui X L, Liu X L, Wong S S, An H, Yan N, Xie J Y, Yu C, Zhang P X, Du Y H, Xi S B, Zheng L R, Cao X Z, Wu Y J, Wang Y X, Wang C Q, Wen H M, Chen L, Xing H B, Wang J. Self-assembled iron-containing mordenite monolith for carbon dioxide sieving[J]. Science, 2021, 373(6552): 315-320.https://doi.org/10.1126/science.aax5776 |
| [21] | Kar S, Kim D, Bin Mohamad Annuar A, Sarma B B, Stanton M, Lam E, Bhattacharjee S, Karak S, Greer H F, Reisner E. Direct air capture of CO2 for solar fuel production in flow[J]. Nat. Energy, 2025, 10: 448-459.https://doi.org/10.1038/s41560-025-01714-y |
| [22] | Liu C X, Ji Y, Zheng T T, Xia C. Solid-state-electrolyte reactor: New opportunity for electrifying manufacture[J]. JACS Au, 2025, 5(2):521-535.https://doi.org/10.1021/jacsau.4c01183 |
| [23] | Chinese Society of Electrochemistry. The top ten scientific questions in electrochemistry[J]. J. Electrochem., 2024, 30(1): 2024121.https://doi.org/10.61558/2993-074x.3444 |
| [24] | Sun J P, Wu B C, Wang Z X, Guo H J, Yan G C, Duan H, Li G C, Wang Y, Wang J X. Rational catalyst layer design enables tailored transport channels for efficient CO2 electrochemical reduction to multi-carbon products[J]. Energy Environ. Sci., 2025, 18(2): 1027-1037.https://doi.org/10.1039/d4ee03743j |
| [25] | Chen H J, Tang M H, Chen S L. Hydrophobicity optimization of cathode catalyst layer for proton exchange membrane fuel cell[J]. J. Electrochem., 2023, 29(9): 2207061.https://doi.org/10.13208/j.electrochem.2207061 |
| [26] | Huang J E, Li F W, Ozden A, Sedighian Rasouli A, García De Arquer F P, Liu S J, Zhang S Z, Luo M C, Wang X, Lum Y W, Xu Y, Bertens K, Miao R K, Dinh C T, Sinton D, Sargent E H. CO2 electrolysis to multicarbon products in strong acid[J]. Science, 2021, 372(6546): 1074-1078.https://doi.org/10.1126/science.abg6582 |
| [27] | Zhang G R, Ye K, Ni B X, Jiang K. Steering the products distribution of CO2 electrolysis: A perspective on extrinsic tuning knobs[J]. Chem Catal., 2023, 3(9): 100746.https://doi.org/10.1016/j.checat.2023.100746 |
| [28] | Yuan S, Wang R Y, Xue R, Wu L Z, Zhang G R, Li H Y, Wang Q, Yin J W, Luo L X, Shen S Y, An L, Yan X H, Zhang J L. Flow field design matters for high current density zero-gap CO2 electrolyzers[J]. ACS Energy Lett., 2024, 9(12): 5945-5954.https://doi.org/10.1021/acsenergylett.4c02534 |
| [29] | Kim C, Bui J C, Luo X Y, Cooper J K, Kusoglu A, Weber A Z, Bell A T. Tailored catalyst microenvironments for CO2 electroreduction to multicarbon products on copper using bilayer ionomer coatings[J]. Nat. Energy, 2021, 6(11): 1026-1034.https://doi.org/10.1038/s41560-021-00920-8 |
| [30] | Nesbitt N T, Burdyny T, Simonson H, Salvatore D, Bohra D, Kas R, Smith W A. Liquid-solid boundaries dominate activity of CO2 reduction on gas-diffusion electrodes[J]. ACS Catal., 2020, 10(23): 14093-14106.https://doi.org/10.1021/acscatal.0c03319 |
| [31] | Fan J, Han N, Li Y G. Electrochemical carbon dioxide reduction in flow cells[J]. J. Electrochem., 2020, 26(4): 510-520.https://doi.org/10.13208/j.electrochem.200443 |
| [32] | Niu Z Z, Gao F Y, Zhang X L, Yang P P, Liu R, Chi L P, Wu Z Z, Qin S, Yu X, Gao M R. Hierarchical copper with inherent hydrophobicity mitigates electrode flooding for high-rate CO2 electroreduction to multicarbon products[J]. J. Am. Chem. Soc., 2021, 143(21): 8011-8021.https://doi.org/10.1021/jacs.1c01190 |
| [33] | Yang K L, Kas R, Smith W A, Burdyny T. Role of the carbon-based gas diffusion layer on flooding in a gas diffusion electrode cell for electrochemical CO2 reduction[J]. ACS Energy Lett., 2020, 6(1): 33-40.https://doi.org/10.1021/acsenergylett.0c02184 |
| [34] | Gabardo C M, O’brien C P, Edwards J P, Mccallum C, Xu Y, Dinh C T, Li J, Sargent E H, Sinton D. Continuous carbon dioxide electroreduction to concentrated multi-carbon products using a membrane electrode assembly[J]. Joule, 2019, 3(11): 2777-2791.https://doi.org/10.1016/j.joule.2019.07.021 |
| [35] | Liu P X, Peng L W, He R N, Li L L, Qiao J L. A high-performance continuous-flow MEA reactor for electroreduction CO2 to formate[J]. J. Electrochem., 2022, 28(1): 2104231.https://doi.org/10.13208/j.electrochem.210423 |
| [36] | Bai Q Q, Xiong L K, Zhang Y J, Ma M T, Jiao Z Y, Lyu F, Deng Z, Peng Y. Salt precipitation and water flooding intrinsic to electrocatalytic CO2 reduction in acidic membrane electrode assemblies: Fundamentals and remedies[J]. EES Catal., 2024, 2(6): 1228-1237.https://doi.org/10.1039/d4ey00170b |
| [37] | Hao S Y, Elgazzar A, Ravi N, Wi T-U, Zhu P, Feng Y G, Xia Y, Chen F-Y, Shan X N, Wang H T. Improving the operational stability of electrochemical CO2 reduction reaction via salt precipitation understanding and management[J]. Nat. Energy, 2025, 10(2): 266-277.https://doi.org/10.1038/s41560-024-01695-4 |
| [38] | Zhu P, Wang H T. High-purity and high-concentration liquid fuels through CO2 electroreduction[J]. Nat. Catal., 2021, 4(11): 943-951.https://doi.org/10.1038/s41929-021-00694-y |
| [39] | Yang H Z, Kaczur J J, Sajjad S D, Masel R I. Electrochemical conversion of CO2 to formic acid utilizing sustainion? membranes[J]. J. CO2 Util., 2017, 20: 208-217.https://doi.org/10.1016/j.jcou.2017.04.011 |
| [40] | Xia C, Xia Y, Zhu P, Fan L, Wang H T. Direct electrosynthesis of pure aqueous H2O2solutions up to 20% by weight using a solid electrolyte[J]. Science, 2019, 366(6462): 226-231.https://doi.org/10.1126/science.aay1844 |
| [41] | Zhu P, Xia C, Liu C Y, Jiang K, Gao G H, Zhang X, Xia Y, Lei Y J, Alshareef H N, Senftle T P, Wang H T. Direct and continuous generation of pure acetic acid solutions via electrocatalytic carbon monoxide reduction[J]. Proc. Natl. Acad. Sci. U S A, 2021, 118(2): e2010868118.https://doi.org/10.1073/pnas.2010868118 |
| [42] | Xia C, Zhu P, Jiang Q, Pan Y, Liang W T, Stavitski E, Alshareef H N, Wang H T. Continuous production of pure liquid fuel solutions via electrocatalytic CO2 reduction using solid-electrolyte devices[J]. Nat. Energy, 2019, 4(9): 776-785.https://doi.org/10.1038/s41560-019-0451-x |
| [43] | Zhang S K, Feng Y G, Elgazzar A, Xia Y, Qiu C, Adler Z, Sellers C, Wang H T. Interfacial electrochemical-chemical reaction coupling for efficient olefin oxidation to glycols[J]. Joule, 2023, 7(8): 1887-1901.https://doi.org/10.1016/j.joule.2023.06.022 |
| [44] | Wi T-U, Xie Y C, Levell Z H, Feng D Y, Kim J Y T, Zhu P, Elgazzar A, Jeon T H, Shakouri M, Hao S Y, Fang Z W, Qiu C, Lee H-W, Hicks A, Liu Y Y, Liu C, Wang H T. Upgrading carbon monoxide to bioplastics via integrated electrochemical reduction and biosynthesis[J]. Nat. Synth., 2024, 3(11): 1392-1403.https://doi.org/10.1038/s44160-024-00621-6 |
| [45] | Wi T-U, Levell Z H, Hao S Y, Elgazzar A, Zhu P, Feng Y G, Chen F Y, Lam W P, Shakouri M, Liu Y Y, Wang H T. Selective and stable ethanol synthesis via electrochemical CO2 reduction in a solid electrolyte reactor[J]. ACS Energy Lett., 2025, 10(2): 822-829.https://doi.org/10.1021/acsenergylett.4c03091 |
| [46] | Kim J Y T, Zhu P, Chen F Y, Wu Z Y, Cullen D A, Wang H T. Recovering carbon losses in CO2 electrolysis using a solid electrolyte reactor[J]. Nat. Catal., 2022, 5(4): 288-299.https://doi.org/10.1038/s41929-022-00763-w |
| [47] | Sanz-Perez E S, Murdock C R, Didas S A, Jones C W. Direct capture of CO2 from ambient air[J]. Chem. Rev., 2016, 116(19): 11840-11876.https://doi.org/10.1021/acs.chemrev.6b00173 |
| [48] | Erans M, Sanz-Perez E S, Hanak D P, Clulow Z, Reiner D M, Mutch G A. Direct air capture: Process technology, techno-economic and socio-political challenges[J]. Energy Environ. Sci., 2022, 15(4): 1360-1405.https://doi.org/10.1039/d1ee03523a |
| [49] | Zeman F S L, K. S. Capturing carbon dioxide directly from the atmosphere[J]. World Res. Rev., 2004, 16: 157-172. https://www.researchgate.net/publication/284761334 |
| [50] | Baciocchi R, Storti G, Mazzotti M. Process design and energy requirements for the capture of carbon dioxide from air[J]. Chem. Eng. Process., 2006, 45(12): 1047-1058.https://doi.org/10.1016/j.cep.2006.03.015 |
| [51] | Chi L P, Niu Z Z, Zhang Y C, Zhang X L, Liao J, Wu Z Z, Yu P C, Fan M H, Tang K B, Gao M R. Efficient and stable acidic CO2 electrolysis to formic acid by a reservoir structure design[J]. Proc. Natl. Acad. Sci. USA, 2023, 120(51): e2312876120.https://doi.org/10.1073/pnas.2312876120 |
| [52] | Zhang Z S, Melo L, Jansonius R P, Habibzadeh F, Grant E R, Berlinguette C P. pH matters when reducing CO2 in an electrochemical flow cell[J]. ACS Energy Lett., 2020, 5(10): 3101-3107.https://doi.org/10.1021/acsenergylett.0c01606 |
| [53] | Petrov K V, Koopman C I, Subramanian S, Koper M T M, Burdyny T, Vermaas D A. Bipolar membranes for intrinsically stable and scalable CO2 electrolysis[J]. Nat. Energy, 2024, 9(8): 932-938.https://doi.org/10.1038/s41560-024-01574-y |
| [54] | Zhu P, Wu Z Y, Elgazzar A, Dong C X, Wi T U, Chen F Y, Xia Y, Feng Y G, Shakouri M, Kim J Y T, Fang Z W, Hatton T A, Wang H T. Continuous carbon capture in an electrochemical solid-electrolyte reactor[J]. Nature, 2023, 618(7967): 959-966.https://doi.org/10.1038/s41586-023-06060-1 |
| [55] | Zhang X, Fang Z W, Zhu P, Xia Y, Wang H T. Electrochemical regeneration of high-purity CO2 from (bi)carbonates in a porous solid electrolyte reactor for efficient carbon capture[J]. Nat. Energy, 2025, 10(1): 55-65.https://doi.org/10.1038/s41560-024-01654-z |
| [56] | Li K, Fan Q, Chuai H Y, Liu H, Zhang S, Ma X B. Revisiting chlor-alkali electrolyzers: From materials to devices[J]. T. Tianjin U., 2021, 27(3): 202-216.https://doi.org/10.1007/s12209-021-00285-9 |
| [57] | Yang J Y, Zhu C X, Li W H, Zheng X S, Wang D S. Organocatalyst supported by a single-atom support accelerates both electrodes used in the chlor-alkali industry via modification of non-covalent interactions[J]. Angew. Chem. Int. Ed., 2024, 63(8): e202314382.https://doi.org/10.1002/anie.202314382 |
| [58] | Jouny M, Luc W, Jiao F. General techno-economic analysis of CO2 electrolysis systems[J]. Ind. Eng. Chem. Res., 2018, 57(6): 2165-2177.https://doi.org/10.1021/acs.iecr.7b03514 |
| [59] | Kibria M G, Edwards J P, Gabardo C M, Dinh C-T, Seifitokaldani A, Sinton D, Sargent E H. Electrochemical CO2 reduction into chemical feedstocks: From mechanistic electrocatalysis models to system design[J]. Adv. Mater., 2019, 31(31): 1807166.https://doi.org/10.1002/adma.201807166 |
| [60] | Jiang T W, Jiang K, Cai W B. Electrochemical CO2 reduction on pd-based electrodes: From mechanism understanding to rational catalyst design[J]. J. Mater. Chem. A, 2024, 12(33): 21515-21530.https://doi.org/10.1039/d4ta02379j |
| [61] | Fan L, Xia C, Zhu P, Lu Y Y, Wang H T. Electrochemical CO2 reduction to high-concentration pure formic acid solutions in an all-solid-state reactor[J]. Nat. Commun., 2020, 11(1): 3633.https://doi.org/10.1038/s41467-020-17403-1 |
| [62] | Zheng T T, Liu C X, Guo C X, Zhang M L, Li X, Jiang Q, Xue W Q, Li H L, Li A W, Pao C W, Xiao J P, Xia C, Zeng J. Copper-catalysed exclusive CO2 to pure formic acid conversion via single-atom alloying[J]. Nat. Nanotechnol., 2021, 16(12): 1386-1393.https://doi.org/10.1038/s41565-021-00974-5 |
| [63] | Zhang G R, Tan B, Mok D H, Liu H Y, Ni B X, Zhao G, Ye K, Huo S J, Miao X H, Liang Z, Liu X, Chen L W, Zhang Z M, Cai W B, Back S, Jiang K. Electrifying hcooh synthesis from CO2 building blocks over Cu-Bi nanorod arrays[J]. Proc. Natl. Acad. Sci. USA, 2024, 121(29): e2400898121.https://doi.org/10.1073/pnas.2400898121 |
| [64] | Zhang G R, Ji N, Lyu S, Ni B X, Shen P, Ye K, Wang Y T, Jiang X H, Zhang H, Liu X, Wang Y C, Jiang K. Artificial synthesis of polyesters at ambient condition via consecutive CO2 electrolysis and fermentation[J]. Nano Res., 2024, 17(7): 6016-6025.https://doi.org/10.1007/s12274-024-6658-6 |
| [65] | Elgazzar A, Zhu P, Chen F Y, Hao S Y, Wi T-U, Qiu C, Okatenko V, Wang H T. Electrochemical CO2 reduction to formic acid with high carbon efficiency[J]. ACS Energy Lett., 2025, 10: 450-458.https://doi.org/10.1021/acsenergylett.4c02773 |
| [66] | Nankya R, Xu Y T, Elgazzar A, Zhu P, Wi T U, Qiu C, Feng Y G, Che F L, Wang H T. Cobalt-doped bismuth nanosheet catalyst for enhanced electrochemical CO2 reduction to electrolyte-free formic acid[J]. Angew. Chem. Int. Ed., 2024, 63(36): e202403671.https://doi.org/10.1002/anie.202403671 |
| [67] | Lin L, He X Y, Zhang X G, Ma W C, Zhang B, Wei D Y, Xie S J, Zhang Q H, Yi X D, Wang Y. A nanocomposite of bismuth clusters and Bi2O2CO3 sheets for highly efficient electrocatalytic reduction of CO2 to formate[J]. Angew. Chem. Int. Ed., 2023, 62(3): e202214959.https://doi.org/10.1002/anie.202214959 |
| [68] | Zhang C, Hao X B, Wang J T, Ding X Y, Zhong Y, Jiang Y W, Wu M C, Long R, Gong W B, Liang C H, Cai W W, Low J X, Xiong Y J. Concentrated formic acid from CO2 electrolysis for directly driving fuel cell[J]. Angew. Chem. Int. Ed., 2024, 63(13): e202317628.https://doi.org/10.1002/anie.202317628 |
| [69] | Zhao Z H, Huang J R, Huang D S, Zhu H L, Liao P Q, Chen X M. Efficient capture and electroreduction of dilute CO2 into highly pure and concentrated formic acid aqueous solution[J]. J. Am. Chem. Soc., 2024, 146(20): 14349-14356.https://doi.org/10.1021/jacs.4c04841 |
| [70] | Wang Z T, Zhou Y S, Liu D Y, Qi R J, Xia C F, Li M T, You B, Xia B Y. Carbon-confined indium oxides for efficient carbon dioxide reduction in a solid-state electrolyte flow cell[J]. Angew. Chem. Int. Ed., 2022, 61(21): e202200552.https://doi.org/10.1002/anie.202200552 |
| [71] | Yang H, Kaczur J J, Sajjad S D, Masel R I. Performance and long-term stability of CO2 conversion to formic acid using a three-compartment electrolyzer design[J]. J. CO2 Util., 2020, 42: 101349.https://doi.org/10.1016/j.jcou.2020.101349 |
| [72] | Zhu H L, Huang J R, Zhang M D, Yu C, Liao P Q, Chen X M. Continuously producing highly concentrated and pure acetic acid aqueous solution via direct electroreduction of CO2[J]. J. Am. Chem. Soc., 2024, 146(1): 1144-1152.https://doi.org/10.1021/jacs.3c12423 |
| [73] | Rutjens B, Von Foerster K, Schmid B, Weinrich H, Sanz S, Tempel H, Eichel R-A. Impact of the piperion anion exchange membrane thickness on the performance of a CO2-to-HCOOH three-compartment electrolyzer[J]. Ind. Eng. Chem. Res., 2024, 63(9): 3986-3996.https://doi.org/10.1021/acs.iecr.3c04459 |
| [74] | Bui J C, Lees E W, Marin D H, Stovall T N, Chen L, Kusoglu A, Nielander A C, Jaramillo T F, Boettcher S W, Bell A T, Weber A Z. Multi-scale physics of bipolar membranes in electrochemical processes[J]. Nat. Chem. Eng., 2024, 1(1): 45-60.https://doi.org/10.1038/s44286-023-00009-x |
| [75] | Zhao E Z, Zhang Y X, Zhan J H, Xia G S, Yu G, Wang Y J. Optimization and scaling-up of porous solid electrolyte electrochemical reactors for hydrogen peroxide electrosynthesis[J]. Nat. Commun., 2025, 16(1): 3212.https://doi.org/10.1038/s41467-025-58385-2 |
| [76] | Zhang X, Xia Y, Xia C, Wang H T. Insights into practical-scale electrochemical H2O2 synthesis[J]. Trends Chem., 2020, 2(10): 942-953.https://doi.org/10.1016/j.trechm.2020.07.007 |
| [77] | Xia Y, Zhao X H, Xia C, Wu Z Y, Zhu P, Kim J Y T, Bai X W, Gao G H, Hu Y F, Zhong J, Liu Y Y, Wang H T. Highly active and selective oxygen reduction to H2O2 on boron-doped carbon for high production rates[J]. Nat. Commun., 2021, 12(1): 4225.https://doi.org/10.1038/s41467-021-24329-9 |
| [78] | Zhang M D, Huang J R, Liang C P, Chen X M, Liao P Q. Continuous electrosynthesis of pure H2O2 solution with medical-grade concentration by a conductive Ni-phthalocyanine-based covalent organic framework[J]. J. Am. Chem. Soc., 2024, 146(45): 31034-31041.https://doi.org/10.1021/jacs.4c10675 |
| [79] | Chen F Y, Elgazzar A, Pecaut S, Qiu C, Feng Y, Ashokkumar S, Yu Z, Sellers C, Hao S Y, Zhu P, Wang H T. Electrochemical nitrate reduction to ammonia with cation shuttling in a solid electrolyte reactor[J]. Nat. Catal., 2024, 7(9): 1032-1043.https://doi.org/10.1038/s41929-024-01200-w |
| [80] | Liu Y C, Huang J R, Zhu H L, Qiu X F, Yu C, Chen X M, Liao P Q. Electrosynthesis of pure urea from pretreated flue gas in a proton-limited environment established in a porous solid-state electrolyte electrolyser[J]. Nat. Nanotechnol., 2025.https://doi.org/10.1038/s41565-025-01914-3 |
/
| 〈 |
|
〉 |