

调控锡氧化物基材料功能特性的新见解
New Insights into Controlling the Functional Properties of Tin Oxide-Based Materials
Received date: 2024-08-26
Revised date: 2024-10-14
Accepted date: 2024-11-12
Online published: 2024-11-14
开发纳米结构材料微观特性和组成可控的制备方法,是发展具有明确功能特性材料制备技术的重要基础。脉冲电解法,一种自上而下的电化学方法,已被证明是制备纳米结构材料的可行方法,对合成锡氧化物基材料尤其有效。这种方法通过改变水性电解质的阴离子成分,可以有效地控制锡氧化物颗粒产物的组成和形状,从而避免在合成过程中使用额外的封端剂,并省去高温后处理步骤。本文结果表明,脉冲电解法得到的锡氧化物的组成和微观结构特性取决于氟化锡和氯化锡络合物不同的稳定性,以及氯化阴离子和氟化阴离子与锡氧化物表面不同的相互作用机制,并受到这些卤素阴离子不同的各向同性/各向混杂性的影响。该方法所获得的分散性锡氧化物的组成和微观结构特征决定了它们作为锂离子电池负极材料、光催化剂或铂基电催化剂混合载体亲氧组分的潜在应用。
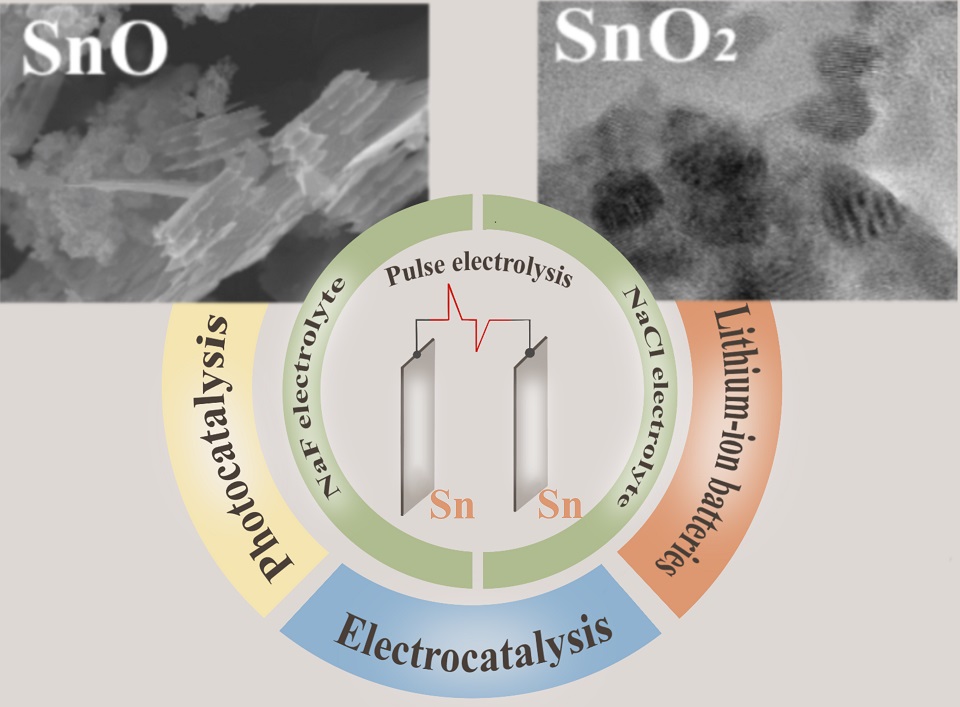
Alexandra Kuriganova , Nina Smirnova . 调控锡氧化物基材料功能特性的新见解[J]. 电化学, 2025 , 31(1) : 2408261 . DOI: 10.61558/2993-074X.3509
Development of methodologies for fabrications of nanostructured materials that provide control over their microstructural features and compositions represents a fundamental step in the advancement of technologies for productions of materials with well-defined functional properties. Pulse electrolysis, a top-down electrochemical approach, has been demonstrated to be a viable method for producing nanostructured materials with a particular efficacy in the synthesis of tin oxides. This method allows for significant control over the composition and shape of the resulting tin oxides particles by modifying the anionic composition of the aqueous electrolyte, obviating the need for additional capping agents in the synthesis process and eliminating the requirement for high-temperature post-treatments. The composition and microstructural characteristics of these oxides are found to be contingent upon the differing stabilities of tin fluoride and chloride complexes, as well as the distinct mechanisms of interaction between chloride and fluoride anions with an oxidized tin surface, which is influenced by the varying kosmotropic/chaotropic nature of these anions. The composition and microstructural characteristics of the obtained dispersed tin oxides would thus determine their potential applications as an anode material for lithium-ion batteries, as a photocatalyst, or as an oxyphilic component of a hybrid support for a platinum-containing electrocatalyst.

Key words: tin oxide; pulse electrolysis; lithium-ion battery; photocatalysis; fuel cell
| [1] | Terna A D, Elemike E E, Mbonu J I, Osafile O E, Ezeani R O. The future of semiconductors nanoparticles: Synthesis, properties and applications[J]. Mater. Sci. Eng. B, 2021, 272: 115363. |
| [2] | Hossain N, Mobarak M H, Mimona M A, Islam M A, Hossain A, Zohura F T, Chowdhury M A. Advances and significances of nanoparticles in semiconductor applications - A review[J]. Results in Engineering, 2023, 19: 101347. |
| [3] | Mishra S R, Ahmaruzzaman M. tin oxide based nanostructured materials: synthesis and potential applications[J]. Nanoscale, 2022, 14(5): 1566-1605. |
| [4] | Yang Y, Guo L P, Wang X P, Li Z Z, Zhou W. Halogen modified organic porous semiconductors in photocatalysis: mechanism, synthesis, and application[J]. Adv. Powder Mater., 2024, 3(2): 100178. |
| [5] | Shabna S, Dhas S S J, Biju C S. Potential progress in SnO2 nanostructures for enhancing photocatalytic degradation of organic pollutants[J]. Catal. Commun., 2023, 177: 106642. |
| [6] | Lan X X, Xiong X Y, Liu J, Yuan B, Hu R Z, Zhu M. Insight into reversible conversion reactions in SnO2-based anodes for lithium storage: a review[J]. Small, 2022, 18(26): 2201110. |
| [7] | Ansari M Z, Ansari S A, Kim S H. Fundamentals and recent progress of Sn-based electrode materials for supercapacitors: A comprehensive review[J]. J. Energy Storage, 2022, 53: 105187. |
| [8] | Chisaka M. Review of carbon-support-free platinum and non-platinum catalysts for polymer electrolyte fuel cells: Will they feature in future vehicles?[J]. J. Mater. Chem. A, 2024, 12: 18615-19582 |
| [9] | Sun C Y, Yang J K, Xu M, Cui Y, Ren W W, Zhang J X, Zhao H L, Liang B. Recent intensification strategies of SnO2-based photocatalysts: A review[J]. Chem. Eng. J., 2022, 427: 131564. |
| [10] | Han J H, Teng X Y, Jia W H, Liu P, Li Y M, Wang W D. SnO2/Fe2O3/Cu2O composites as catalysts for photoelectrocatalytic degradation of benzotriazoles[J]. Opt. Mater., 2024, 148: 114799. |
| [11] | Inaba M, Murase R, Takeshita T, Yano K, Kosaka S, Takahashi N, Isomura N, Oh-ishi K, Yoshimune W, Tsuchiya K, Nobukawa T, Kodama K. Synthesis of a mesoporous SnO2catalyst support and the effect of its pore size on the performance of polymer electrolyte fuel cells[J]. ACS Appl. Mater. Interfaces, 2024, 16(8): 10295-19306. |
| [12] | Guan M M, Zhao X R, Duan L B, Cao M M, Guo W R, Liu J R, Zhang W. Controlled synthesis of SnO2nanostructures with different morphologies and the influence on photocatalysis properties[J]. J. Appl. Phys., 2013, 114(11): 114302. |
| [13] | Periyasamy M, Kar A. Modulating the properties of SnO2 nanocrystals: morphological effects on structural, photoluminescence, photocatalytic, electrochemical and gas sensing properties[J]. J. Mater. Chem. C, 2020, 8(14): 4604-4635. |
| [14] | Habte A G, Hone F G, Dejene F B. Effect of solution pH on structural, optical and morphological properties of SnO2 nanoparticles[J]. Physica B Condens. Matter, 2020, 580: 411832. |
| [15] | Sun X L. Morphosynthesis of SnO2 nanocrystal networks as high-capacity anodes for lithium ion batteries[J]. Ionics, 2020, 26(8): 3841-3851. |
| [16] | Wang H, Rogach A L. Hierarchical SnO2 nanostructures: Recent advances in design, synthesis, and applications[J]. Chem. Mater., 2014, 26(1): 123-133. |
| [17] | Kuriganova A, Faddeev N, Gorshenkov M, Kuznetsov D, Leontyev I, Smirnova N. A comparison of “bottom-up” and “top-down” approaches to the synthesis of Pt/C electrocatalysts[J]. Processes, 2020, 8(8): 947. |
| [18] | Leontyev I, Kuriganova A, Kudryavtsev Y, Dkhil B, Smirnova N. New life of a forgotten method: Electrochemical route toward highly efficient Pt/C catalysts for low-temperature fuel cells[J]. Appl. Catal., A, 2012, 431: 120-125. |
| [19] | Faddeev N A, Kuriganova A B, Leont’ev I N, Smirnova N V. Palladium-based electroactive materials for environmental catalysis[J]. Dokl. Phys. Chem., 2022, 507(1): 139-146. |
| [20] | Kuriganova A B, Faddeev N A, Leontyev I N, Allix M, Rakhmatullin A, Smirnova N V. New electrochemical approach for the synthesis of Pd-PdO/C electrocatalyst and application to formic acid electrooxidation[J]. ChemistrySelect, 2019, 4(29): 8390-8393. |
| [21] | Leontyeva D V, Leontyev I N, Avramenko M V, Yuzyuk Y I, Kukushkina Y A, Smirnova N V. Electrochemical dispergation as a simple and effective technique toward preparation of NiO based nanocomposite for supercapacitor application[J]. Electrochim. Acta, 2013, 114: 356-362. |
| [22] | Chernysheva D, Vlaic C, Leontyev I, Pudova L, Ivanov S, Avramenko M, Allix M, Rakhmatullin A, Maslova O, Bund A, Smirnova N. Synthesis of Co3O4/CoOOH via electrochemical dispersion using a pulse alternating current method for lithium-ion batteries and supercapacitors[J]. Solid State Sci., 2018, 86: 53-59. |
| [23] | Molodtsova T, Gorshenkov M, Kubrin S, Saraev A, Ulyankina A, Smirnova N. One-step access to bifunctional γ-Fe2O3/δ-FeOOH electrocatalyst for oxygen reduction reaction and acetaminophen sensing[J]. J. Taiwan Inst. Chem. Eng., 2022, 140: 104569. |
| [24] | Molodtsova T, Gorshenkov M, Saliev A, Vanyushin V, Goncharov I, Smirnova N. One-step synthesis of γ-Fe2O3/Fe3O4 nanocomposite for sensitive electrochemical detection of hydrogen peroxide[J]. Electrochim. Acta, 2021, 370: 137723. |
| [25] | Ulyankina A, Leontyev I, Maslova O, Allix M, Rakhmatullin A, Nevzorova N, Valeev R, Yalovega G, Smirnova N. Copper oxides for energy storage application: Novel pulse alternating current synthesis[J]. Mater. Sci. Semicond. Process, 2018, 731: 11-16. |
| [26] | Ulyankina A, Leontyev I, Avramenko M, Zhigunov D, Smirnova N. Large-scale synthesis of ZnO nanostructures by pulse electrochemical method and their photocatalytic properties[J]. Mater. Sci. Semicond. Process, 2018, 76: 7-13. |
| [27] | Ulyankina A, Molodtsova T, Gorshenkov M, Leontyev I, Zhigunov D, Konstantinova E, Lastovina T, Tolasz J, Henych J, Licciardello N, Cuniberti G, Smirnova N. Photocatalytic degradation of ciprofloxacin in water at nano-ZnO prepared by pulse alternating current electrochemical synthesis[J]. J. Water Process. Eng., 2021, 40: 101809. |
| [28] | Ulyankina A, Tsarenko A, Yatsenko A, Gorshenkov M, Smirnova N. Photo(electro)catalytic performance of ZnO-based nanopowders prepared through pulse alternating current electrosynthesis in alkaline earth chloride electrolytes[J]. ChemistrySelect, 2023, 8(37): e202300457. |
| [29] | Tsarenko A, Gorshenkov M, Yatsenko A, Zhigunov D, Butova V, Kaichev V, Ulyankina A. Electrochemical synthesis-dependent photoelectrochemical properties of tungsten oxide powders[J]. Chemengineering, 2022, 6(2): 31. |
| [30] | Ulyankina A, Tsarenko A, Molodtsova T, Yatsenko A, Gorshenkov M, Kaichev V, Kuriganova A, Smirnova N. Tungsten oxide nanopowders: pulse alternating current electrosynthesis, structure optimization and performance in a flow photocatalytic fuel cell[J]. J. Mater. Sci., 2023, 58(27): 11187-11197. |
| [31] | Ulyankina A, Avramenko M, Kusnetsov D, Firestein K, Zhigunov D, Smirnova N. Electrochemical synthesis of TiO2 under pulse alternating current: effect of thermal treatment on the photocatalytic activity[J]. ChemistrySelect, 2019, 4(6): 2001-2007. |
| [32] | Molodtsova T A, Kuriganova A B, Ivanov L N, Leontyev I N, Smirnova N V. Optimization of c/rh-In2O3-based electrode technology for photoelectrochemical systems[J]. Kinet. Catal., 2024, 65(5): 597-604. |
| [33] | Ulyankina A A, Kuriganova A B, Smirnova N V. Photocatalytic properties of SnO2-SnO nanocomposite prepared via pulse alternating current synthesis[J]. Mendeleev Commun., 2019, 29(2): 215-217. |
| [34] | Molodtsova T, Gorshenkov M, Kolesnikov E, Leontyev I, Kaichev V, Zhigunov D, Faddeev N, Kuriganova A, Smirnova N. Fabrication of nano-In2O3 phase junction by pulse alternating current synthesis for enhanced photoelectrochemical performance: Unravelling the role of synthetic conditions[J]. Ceramics Int., 2023, 49(7): 10986-10992. |
| [35] | Kuriganova A B, Smirnova N V, Leontyev I N, Avramenko M V. Investigation of structural, microstructural and electrochemical characteristics of Pt/SnOx-C electrocatalysts prepared via electrochemical dispersion of tin and platinum[J]. ChemChemTech, 2019, 62(9): 53-59. |
| [36] | Kuriganova A B, Leontyev I N, Avramenko M V, Faddeev N A, Smirnova N V. Graphene structures prepared via pulse alternating current technique[J]. Mendeleev Commun., 2022, 32(3): 308-310. |
| [37] | Kuriganova A B, Leontyev I N, Avramenko M V, Popov Y, Maslova O A, Koval O Y, Smirnova N V. One-step simultaneous synthesis of graphene and Pt nanoparticles under the action of pulsed alternating current and electrochemical performance of Pt/graphene catalysts[J]. ChemistrySelect, 2017, 2(24): 6979-6983. |
| [38] | Kuriganova A, Kubanova M, Leontyev I, Molodtsova T, Smirnova N. Pulse electrolysis technique for preparation of bimetal tin-containing electrocatalytic materials[J]. Catalysts, 2022, 12(11): 1444. |
| [39] | Kuriganova A, Leontyeva D, Ivanov S, Bund A, Smirnova N. Electrochemical dispersion technique for preparation of hybrid MOx-C supports and Pt/MOx-C electrocatalysts for low-temperature fuel cells[J]. J. Appl. Electrochem., 2016, 46(12): 1245-1260. |
| [40] | Kuriganova A B, Smirnova N V. Pt/SnOx-C composite material for electrocatalysis[J]. Mendeleev Commun., 2014, 6(24): 351-352. |
| [41] | Rudi S, Cui C, Gan L, Strasser P. Comparative study of the electrocatalytically active surface areas (ECSAs) of Pt alloy nanoparticles evaluated by hupd and CO-stripping voltammetry[J]. Electrocatal., 2014, 5(4): 408-418. |
| [42] | Metiko?-Hukovi? M, Omanovi? S. Kinetics of anodic oxide-film growth on tin: ionic transport across a barrier in the high-field limit[J]. Mater. Chem. Phys., 1994, 38(1): 55-62. |
| [43] | Palacios-Padrós A, Caballero-Briones F, Díez-Pérez I, Sanz F. Tin passivation in alkaline media: Formation of SnO microcrystals as hydroxyl etching product[J]. Electrochim. Acta, 2013, 111: 837-845. |
| [44] | Kuriganova A B, Brink I Y, Smirnova N. Theoretical and technological fundamentals of pulse electrolysis for the production of electro- and catalytically active materials based on Pt, Pd, Sn and graphene nanostructures[J]. Nano Mater. Sci., 2024, https://doi.org/10.1016/j.nanoms.2024.09.007. |
| [45] | Abrahams I, Grimes S M, Johnston S R, Knowles J C. Ti (II) oxyhydroxide by X-ray powder diffraction[J]. Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1996, 52(2): 286-288. |
| [46] | Tian F, Wang X B, Chen Z Y, Guo Y M, Liang H J, Lu Z S, Wang D, Lou X, Yang L. A facile post-process method to enhance crystallinity and electrochemical properties of SnO2/rGO composites with three-dimensional hierarchically porous structure[J]. RSC Adv., 2016, 6(108): 106275-106284. |
| [47] | Kim W J, Lee S W, Sohn Y. Metallic Sn spheres and SnO2@C core-shells by anaerobic and aerobic catalytic ethanol and CO oxidation reactions over SnO2 nanoparticles[J]. Sci. Rep., 2015, 5: 513448. |
| [48] | Thommes M, Kaneko K, Neimark Alexander V, Olivier James P, Rodriguez-Reinoso F, Rouquerol J, Sing Kenneth S W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report)[J]. Pure Appl. Chem., 2015, 87(9-10): 1051-1069. |
| [49] | Xin F, Whittingham M S. Challenges and development of tin-based anode with high volumetric capacity for Li-ion batteries[J]. Electrochem. Energy Rev., 2020, 3(4): 643-655. |
| [50] | Kuriganova A B, Vlaic C A, Ivanov S, Leontyeva D V, Bund A, Smirnova N V. Electrochemical dispersion method for the synthesis of SnO2 as anode material for lithium ion batteries[J]. J. Appl. Electrochem., 2016, 46(5): 527-538. |
| [51] | Shin J H, Song J Y. Electrochemical properties of Sn-decorated SnO nanobranches as an anode of Li-ion battery[J]. Nano Converg., 2016, 3(1): 9. |
| [52] | Long Z Q, Li Q G, Wei T, Zhang G M, Ren Z J. Historical development and prospects of photocatalysts for pollutant removal in water[J]. J. Hazard. Mater., 2020, 395: 122599. |
| [53] | Byrne C, Subramanian G, Pillai S C. Recent advances in photocatalysis for environmental applications[J]. J. Environ. Chem. Eng., 2018, 6(3): 3531-3555. |
| [54] | Santhi K, Rani C, Karuppuchamy S. Synthesis and characterization of a novel SnO/SnO2 hybrid photocatalyst[J]. J. Alloys Compd., 2016, 662: 102-107. |
| [55] | Roy A, Arbuj S, Waghadkar Y, Shinde M, Umarji G, Rane S, Patil K, Gosavi S, Chauhan R. Concurrent synthesis of SnO/SnO2nanocomposites and their enhanced photocatalytic activity[J]. J. Solid State Electrochem., 2017, 21(1): 9-17. |
| [56] | Bhattacharjee A, Ahmaruzzaman M, Devi T B, Nath J. Photodegradation of methyl violet 6B and methylene blue using tin-oxide nanoparticles (synthesized via a green route)[J]. J. Photochem. Photobiol. A, 2016, 325: 116-124. |
| [57] | Abdelkader E, Nadjia L, Naceur B, Noureddine B. SnO2 foam grain-shaped nanoparticles: Synthesis, characterization and UVA light induced photocatalysis[J]. J. Alloys Compd., 2016, 679: 408-419. |
| [58] | Lin J J, Luo Z Z, Liu J J, Li P. Photocatalytic degradation of methylene blue in aqueous solution by using ZnO-SnO2 nanocomposites[J]. Mater. Sci. Semicond. Process, 2018, 87: 24-31. |
| [59] | Wasmus S, Küver A. Methanol oxidation and direct methanol fuel cells: a selective review[J]. J. Electroanal. Chem., 1999, 461(1-2): 14-31. |
| [60] | Munjewar S S, Thombre S B, Mallick R K. Approaches to overcome the barrier issues of passive direct methanol fuel cell-Review[J]. Renew. Sust. Energ. Rev., 2017, 67: 1087-1104. |
| [61] | Alias M, Kamarudin S, Zainoodin A, Masdar M. Active direct methanol fuel cell: An overview[J]. Int. J. Hydrogen Energy, 2020, 45(38): 19620-19641. |
| [62] | Kamarudin M, Kamarudin S, Masdar M, Daud W. Direct ethanol fuel cells[J]. Int. J. Hydrogen Energy, 2013, 38(22): 9438-9453. |
| [63] | Badwal S, Giddey S, Kulkarni A, Goel J, Basu S. Direct ethanol fuel cells for transport and stationary applications-A comprehensive review[J]. Appl. Energy, 2015, 145: 80-103. |
| [64] | Mussatto S I, Dragone G, Guimar?es P M R, Silva J P A, Carneiro L M, Roberto I C, Vicente A, Domingues L, Teixeira J A. Technological trends, global market, and challenges of bio-ethanol production[J]. Biotechnol. Adv., 2010, 28(6): 817-830. |
| [65] | Willsau J, Heitbaum J. Elementary steps of ethanol oxidation on Pt in sulfuric acid as evidenced by isotope labelling[J]. J. Electroanal. Chem. Interfacial Electrochem., 1985, 194(1): 27-35. |
| [66] | Iwasita T, Pastor E. A DEMS and FTIR spectroscopic investigation of adsorbed ethanol on polycrystalline platinum[J]. Electrochim. Acta, 1994, 39(4): 531-537. |
| [67] | Bittins‐Cattaneo B, Wilhelm S, Cattaneo E, Buschmann H, Vielstich W. Intermediates and products of ethanol oxidation on platinum in acid solution[J]. Ber. Bunsenges. Phys. Chem., 1988, 92(11): 1210-1218. |
| [68] | Hitmi H, Belgsir E, Léger J M, Lamy C, Lezna R. A kinetic analysis of the electro-oxidation of ethanol at a platinum electrode in acid medium[J]. Electrochim. Acta, 1994, 39(3): 407-415. |
| [69] | Gootzen J, Visscher W, Van Veen J. Characterization of ethanol and 1, 2-ethanediol adsorbates on platinized platinum with Fourier transform infrared spectroscopy and differential electrochemical mass spectrometry[J]. Langmuir, 1996, 12(21): 5076-5082. |
| [70] | Schmidt V M, Ianniello R, Pastor E, González S. Electrochemical reactivity of ethanol on porous Pt and PtRu: Oxidation/reduction reactions in 1 M HClO4[J] .J. Phys. Chem., 1996, 100(45): 17901-17908. |
| [71] | Marinkovic N S, Li M, Adzic R R. Pt-based catalysts for electrochemical oxidation of ethanol[J]. Top Curr. Chem. (Z), 2019, 377, 11: 1-39. |
| [72] | Huang H, Blackman O F, Celorrio V, Russell A E. Isolating the contributions of surface Sn atoms in the bifunctional behaviour of PtSn CO oxidation electrocatalysts[J]. Electrochim. Acta, 2021, 390: 138811. |
| [73] | Kuriganova A, Leontyev I, Leontyev N, Smirnova N. Pt Catalysts prepared via top-down electrochemical approach: synthesis methodology and support effects[J]. J. Electrochem. Sci. Technol., 2024, 15(3): 345-352. |
| [74] | Díaz R, Díez-Pérez I, Gorostiza P, Sanz F, Morante J R. An electrochemical study of tin oxide thin film in borate buffer solutions[J]. J. Braz. Chem. Soc., 2003, 14(4): 523-529. |
| [75] | Abd El Wahae F, Abd El Kader J, El Shayeb H, El Din A S. On the pitting corrosion of tin in aqueous solutions[J]. J. Corrosion Science, 1978, 18(11): 997-1009. |
| [76] | Morgan Tench D, Anderson D, Kim P. Solderability assessment via sequential electrochemical reduction analysis[J]. J. Appl. Electrochem., 1994, 24(1): 18-29. |
| [77] | Cho S, Yu J, Kang S K, Shih D Y. Oxidation study of pure tin and its alloys via electrochemical reduction analysis[J]. J. Electron. Mater., 2005, 34(5): 635-642. |
| [78] | Alvarez P, Ribotta S, Folquer M, Gervasi C, Vilche J. Potentiodynamic behaviour of tin in different buffer solutions[J]. Corrosion Sci., 2002, 44(1) :49-65. |
| [79] | Begum S N, Basha A, Muralidharan V, Lee C W. Electrochemical behaviour of tin in alkali solutions containing halides[J]. Mater. Chem. Phys., 2012, 132(2-3): 1048-1052. |
| [80] | Trompette J L. The comparative breakdown of passivity of tin by fluorides and chlorides interpreted through the ‘law of matching affinities’ concept[J]. Corrosion Sci., 2015, 942: 88-93. |
| [81] | Collins K D, Neilson G W, Enderby J E. Ions in water: Characterizing the forces that control chemical processes and biological structure[J]. Biophys. Chem., 2007, 128(2): 95-104. |
| [82] | Collins K D. Ions from the Hofmeister series and osmolytes: effects on proteins in solution and in the crystallization process[J]. Methods, 2004, 34(3): 300-311. |
| [83] | Perrin D D, Dempsey B, Serjeant E P. pKa prediction for organic acids and bases[M]. Springer, 1981,Vol. 1. |
| [84] | Ghosh S, Roy S. Effect of ageing on Sn6O4(OH)4 in aqueous medium—simultaneous production of SnO and SnO2 nanoparticles at room temperature[J]. J. Solgel Sci. Technol., 2017, 81(3): 769-773. |
| [85] | Marcus Y. Thermodynamics of solvation of ions. Part 5. Gibbs free energy of hydration at 298.15 K[J]. J. Chem. Soc., Faraday trans., 1991, 87(18): 2995-2999. |
| [86] | Pauling L. The nature of the chemica bond. IV. The energy of single bonds and the relative electronegativity of atoms[J]. J. Am. Chem. Soc., 1932, 54(9): 3570-3582. |
| [87] | Nakashima T., Fujishima A. Adsorption of anionic and cationic dyes in dilute solutions onto a SnO2 surface measured by total internal reflection fluorescence[J]. Ber. Bunsenges. Phys. Chem., 1995, 99: 609-616. |
| [88] | Séby F, Potin-Gautier M, Giffaut E, Donard O F X. A critical review of thermodynamic data for inorganic tin species[J]. Geochim. Cosmochim. Acta, 2001, 65(18): 3041-3053. |
/
| 〈 |
|
〉 |