

微纳米尺度两互不相溶电解质溶液界面的单颗粒碰撞电化学
收稿日期: 2024-06-10
录用日期: 2024-09-04
网络出版日期: 2024-09-19
Single-Entity Collisional Electrochemistry at the Micro- and/or Nano-Interface Between Two Immiscible Electrolyte Solutions
Received date: 2024-06-10
Accepted date: 2024-09-04
Online published: 2024-09-19
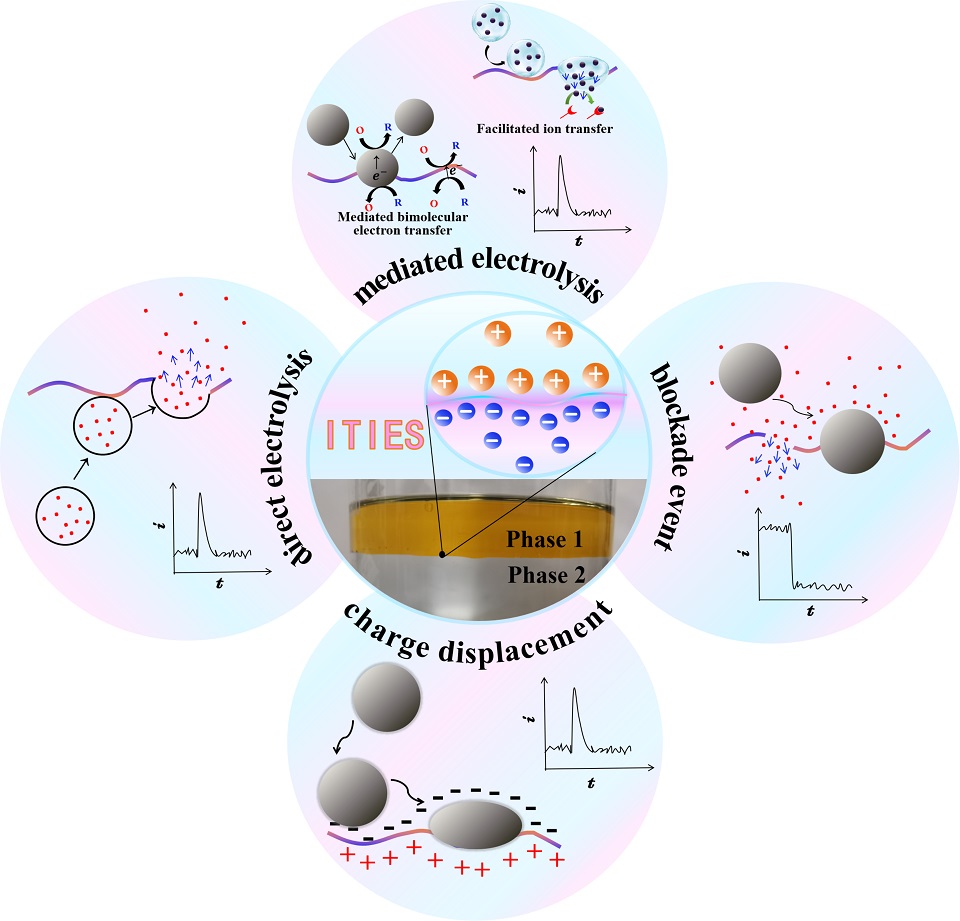
单颗粒碰撞电化学通过溶液中颗粒与电极的随机碰撞,以单颗粒分辨率直接表征实体/颗粒,获得丰富的物理化学信息,成为近二十年来电分析化学的前沿之一。有趣的是,(微/纳米级)传感电极从可极化的液/液(汞/液)界面发展到固/液界面,再到液/液界面(即两互不相溶电解质溶液界面,ITIES),仿佛完成了一个循环(但实际上并没有)。ITIES凭借其可极化性(在水/α,α,α-三氟甲苯界面处高达1.1 V的电势窗口)和高重现性,已成为蓬勃发展的SECE中最新的传感电极。SECE在固/液界面发展起来的四种测量模式(直接电解、介导电解、电流屏蔽和电荷置换)也在微型ITIES上得到了充分实现。本文将从基本概念、运行机制和最新进展(例如离子体的发现、法拉第离子转移的屏蔽效应等)的角度讨论ITIES中的这四种模式,并展望这一新兴领域未来的发展方向。
关键词: 单颗粒碰撞电化学; 两互不相溶电解质溶液界面; 电荷转移
杨丽芳 , 陈俊杰 , 陈灵玉 , 金思琪 , 方韬雄 , 何思佳 , 沈粮骏 , 黄新建 , 孙霄航 , 邓海强 . 微纳米尺度两互不相溶电解质溶液界面的单颗粒碰撞电化学[J]. 电化学, 2024 , 30(11) : 2414005 . DOI: 10.61558/2993-074X.3495
Single-entity collisional electrochemistry (SECE) is a branch of single-entity electrochemistry. It can directly characterize entities/particles with single particle resolution through random collisions between particles and electrodes in a solution, and obtain rich physicochemical information, thus becoming one of the frontiers of electroanalytical chemistry in the past two decades. Interestingly, the (micro/nanoscale) sensing electrodes have evolved from a polarizable liquid/liquid (mercury/liquid) interface to a solid/liquid interface and then to a liquid/liquid interface (i.e., an interface between two immiscible electrolyte solutions, ITIES), as if they have completed a cycle (but in fact they have not). ITIES has become the latest sensing electrode in the booming SECE due to its polarizability (up to 1.1 V at the water/α,α,α-trifluorotoluene interface) and high reproducibility. The four measurement modes (direct electrolysis, mediated electrolysis, current blockade, and charge displacement) developed in the realm of SECE at solid/liquid interfaces have also been fully realized at the miniature ITIES. This article will discuss these four modes at the ITIES from the perspectives of basic concepts, operating mechanisms, and latest developments (e.g., discovery of ionosomes, blockade effect of Faradaic ion transfer, etc.), and look forward to the future development and direction of this emerging field.

/
| 〈 |
|
〉 |