

基于铂纳米颗粒碰撞电化学用于快速检测乳腺癌MCF-7细胞
收稿日期: 2024-04-29
录用日期: 2024-06-08
网络出版日期: 2024-07-09
版权
Platinum Nanoparticle-Based Collision Electrochemistry for Rapid Detection of Breast Cancer MCF-7 Cells
Received date: 2024-04-29
Accepted date: 2024-06-08
Online published: 2024-07-09
Copyright
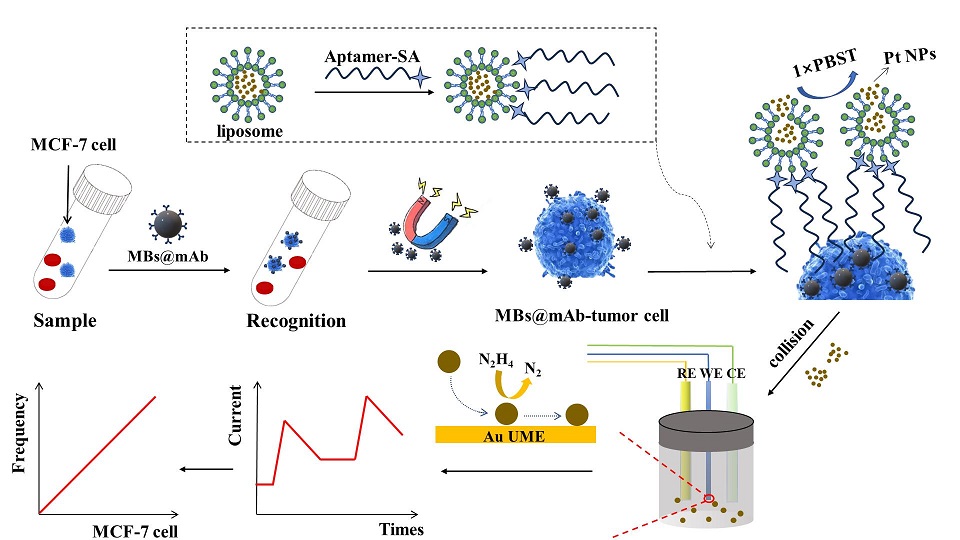
癌症转移是全球癌症患者的主要死因,也是治疗癌症的主要挑战之一。循环肿瘤细胞(CTCs)在癌症转移过程中起着核心作用。但是,CTCs在外周血中的含量极少,在实际样本中检测CTCs极具挑战性,故高效富集和早期检测CTCs对于及时诊断疾病至关重要。本工作利用免疫磁分离技术和脂质体信号放大策略构建了一种创新的、精密的用于检测MCF-7细胞(人类乳腺癌细胞)的SNCE生物传感器。以包埋铂纳米颗粒(Pt NPs)的脂质体为信号探针,以自制的金超微电极(Au UME)为工作电极。Pt NPs与UME的每次有效碰撞都会产生可区分的阶梯型电流。根据细胞浓度与碰撞频率(单位时间内阶梯型电流数量)之间的关系,对MCF-7细胞进行了精确定量,实现了对MCF-7细胞的高灵敏度和特异性检测。该SNCE生物传感器线性范围为10 cells·mL-1至105 cells·mL-1,检测限低至5 cells·mL-1。此外,在复杂样本中成功检测到MCF-7细胞,表面SNCE生物传感器在患者样本检测方面具有巨大潜力。
秦富星 , 李明珂 , 周汇龙 , 文为 , 张修华 , 王升富 , 伍珍 . 基于铂纳米颗粒碰撞电化学用于快速检测乳腺癌MCF-7细胞[J]. 电化学, 2024 , 30(10) : 2414004 . DOI: 10.61558/2993-074X.3483
Cancer metastasis is the leading cause of death in cancer patients worldwide and one of the major challenges in treating cancer. Circulating tumor cells (CTCs) play a pivotal role in cancer metastasis. However, the content of CTCs in peripheral blood is minimal, so the detection of CTCs in real samples is extremely challenging. Therefore, efficient enrichment and early detection of CTCs are essential to achieve timely diagnosis of diseases. In this work, we constructed an innovative and sensitive single-nanoparticle collision electrochemistry (SNCE) biosensor for the detection of MCF-7 cells (human breast cancer cells) by immunomagnetic separation technique and liposome signal amplification strategy. Liposomes embedded with platinum nanoparticles (Pt NPs) were used as signal probes, and homemade gold ultramicroelectrodes (Au UME) were used as the working electrodes. The effective collision between Pt NPs and UME would produce distinguishable step-type current. MCF-7 cells were accurately quantified according to the relationship between cell concentration and collision frequency (the number of step-type currents generated per unit time), realizing highly sensitive and specific detection of MCF-7 cells. The SNCE biosensor has a linear range of 10 cells·mL-1 to 105 cells·mL-1 with a detection limit as low as 5 cells·mL-1. In addition, the successful detection of MCF-7 cells in complex samples showed that the SNCE biosensors have great potential for patient sample detection.

| [1] | Xu W, Zou G Q, Hou H S, Ji X. Single particle electrochemistry of collision[J]. Small, 2019, 15(32): 1804908. |
| [2] | Chen M, Lu S M, Wang H W, Long Y T. Tracking light-induced fragmentation of single silver nanoparticles by single entity electrochemistry[J]. J. Electrochem., 2022, 28(3): 2108521. |
| [3] | Sun L L, Wang W, Chen H Y. Dynamic nanoparticle‐substrate contacts regulate multi‐peak behavior of single silver nanoparticle collisions[J]. ChemElectroChem, 2018, 5(20): 2995-2999. |
| [4] | Oja S M, Robinson D A, Vitti N J, Edwards M A, Liu Y W, White H S, Zhang B. Observation of multipeak collision behavior during the electro-oxidation of single Ag nanoparticles[J]. J. Am. Chem. Soc., 2017, 139(2): 708-718. |
| [5] | Sun L L, Wang W, Chen H Y. Correlated optical imaging and electrochemical recording for studying single nanoparticle collisions[J]. J. Electrochem., 2019, 25(3): 386-399. |
| [6] | Defnet P A, Zhang B. Collision, adhesion, and oxidation of single Ag nanoparticles on a polysulfide-modified microelectrode[J]. J. Am. Chem. Soc., 2021, 143(39): 16154-16162. |
| [7] | Ding Q D, Sun Z H, Ma W. Probing conformational kinetics of catalase with and without magnetic field by single-entity collision electrochemistry[J]. Sci. Bull., 2023, 68(21): 2564-2573. |
| [8] | Zhou M, Wang D, Mirkin M V. Electrochemical evaluation of the number of Au atoms in polymeric gold thiolates by single particle collisions[J]. Anal. Chem., 2018, 90(14): 8285-8289. |
| [9] | Su T, Guo J, He Z K, Zhao J J, Gao Z D, Song Y Y. Single-nanoparticle-level understanding of oxidase-like activity of au nanoparticles on polymer nanobrush-based proton reservoirs[J]. Anal. Chem., 2023, 95(31): 11807-11814. |
| [10] | Guo J, Pan J, Chang S, Wang X W, Kong N, Yang W R, He J. Monitoring the dynamic process of formation of plasmonic molecular junctions during single nanoparticle collisions[J]. Small, 2018, 14(15): 1704164. |
| [11] | Hafez M E, Ma H, Ma W, Long Y T. Unveiling the intrinsic catalytic activities of single‐gold‐nanoparticle‐based enzyme mimetics[J]. Angew. Chem. Int. Ed., 2019, 131(19): 6393-6398. |
| [12] | Bai Y Y, Yang Y J, Xu Y, Yang X Y, Zhang Z L. Current lifetime of single-nanoparticle electrochemical collision for in situ monitoring nanoparticles agglomeration and aggregation[J]. Anal. Chem., 2023, 95(9): 4429-4434. |
| [13] | Zhang J H, Zhou Y G. Single particle impact electrochemistry: analyses of nanoparticles and biomolecules[J]. J. Electrochem., 2019, 25(3): 374-385. |
| [14] | Wang H, Yang C, Tang H, Li Y X. Stochastic collision electrochemistry from single G-quadruplex/hemin: electrochemical amplification and microRNA sensing[J]. Anal. Chem., 2021, 93(10): 4593-4600. |
| [15] | Dunevall J, Fathali H, Najafinobar N, Lovric J, Wigstrom J, Cans C S, Ewing A G. Characterizing the catecholamine content of single mammalian vesicles by collision-adsorption events at an electrode[J]. J. Am. Chem. Soc., 2015, 137(13): 4344-4346. |
| [16] | Dick J E. Electrochemical detection of single cancer and healthy cell collisions on a microelectrode[J]. Chem. Commun., 2016, 52(72): 10906-10909. |
| [17] | Qiu X, Dai Q S, Tang H R, Li Y X. Multiplex assays of MicroRNAs by using single particle electrochemical collision in a single run[J]. Anal. Chem., 2023, 95(35): 13376-13384. |
| [18] | Peng M H, Zhou Y G. Impact electrochemical analysis of soft bio-particles: A mini review[J]. Electrochem. Commun., 2023, 150: 107490. |
| [19] | Fosdick S E, Anderson M J, Nettleton E G, Crooks R M. Correlated electrochemical and optical tracking of discrete collision events[J]. J. Am. Chem. Soc., 2013, 135(16): 5994-5997. |
| [20] | Dick J E, Hilterbrand A T, Strawsine L M, Bard A J. Enzymatically enhanced collisions on ultramicroelectrodes for specific and rapid detection of individual viruses[J]. PNAS, 2016, 113(23): 6403-6408. |
| [21] | Dick J E, Renault C, Bard A J. Observation of single-protein and DNA macromolecule collisions on ultramicroelectrodes[J]. J. Am. Chem. Soc., 2015, 137(26): 8376-8379. |
| [22] | Deng Z, Elattar R, Maroun F, Renault C. In situ measurement of the size distribution and concentration of insulating particles by electrochemical collision on hemispherical ultramicroelectrodes[J]. Anal. Chem., 2018, 90(21): 12923-12929. |
| [23] | Ho T L T, Hoang N T T, Lee J, Park J H, Kim B K. Determining mean corpuscular volume and red blood cell count using electrochemical collision events[J]. Biosens. Bioelectron., 2018, 110: 155-159. |
| [24] | Alix‐Panabières C, Pantel K. Characterization of single circulating tumor cells[J]. FEBS letters, 2017, 591(15): 2241-2250. |
| [25] | Edd J F, Mishra A, Smith K C, Kapur R, Maheswaran S, Haber D A, Toner M. Isolation of circulating tumor cells[J]. Iscience, 2022, 25(8): 104696. |
| [26] | Shen Z Y, Wu A G, Chen X Y. Current detection technologies for circulating tumor cells[J]. Chem. Soc. Rev., 2017, 46(8): 2038-2056. |
| [27] | Ferreira M M, Ramani V C, Jeffrey S S. Circulating tumor cell technologies[J]. Mol. Oncol., 2016, 10(3): 374-394. |
| [28] | Rawal S, Yang Y P, Cote R, Agarwal A. Identification and quantitation of circulating tumor cells[J]. Annu. Rev. Anal. Chem., 2017, 10: 321-343. |
| [29] | Lawrence R, Watters M, Davies C R, Pantel K, Lu Y J. Circulating tumor cells for early detection of clinically relevant cancer[J]. Nat. Rev. Clin. Oncol., 2023, 20(7): 487-500. |
| [30] | Bankó P, Lee S Y, Nagygy?rgy V, Zrínyi M, Chae C H, Cho D H, Telekes A. Technologies for circulating tumor cell separation from whole blood[J]. J. Hematol. Oncol., 2019, 12: 1-20. |
| [31] | Ju S W, Chen C, Zhang J H, Xu L, Zhang X, Li Z Q, Chen Y X, Zhou J C, Ji F Y, Wang L B. Detection of circulating tumor cells: opportunities and challenges[J]. Biomark. Res., 2022, 10(1): 58. |
| [32] | Moon D H, Lindsay D P, Hong S, Wang A Z. Clinical indications for, and the future of, circulating tumor cells[J]. Adv. Drug. Deliver. Rev., 2018, 125: 143-150. |
| [33] | Tretyakova M S, Menyailo M E, Schegoleva A A, Bokova U A, Larionova I V, Denisov E V. Technologies for viable circulating tumor cell isolation[J]. Int. J. Mol. Sci., 2022, 23(24): 15979. |
| [34] | Feng Z X, Wu J Y, Lu Y J, Chan Y T, Zhang C, Wang D, Luo D, Huang Y, Feng Y B, Wang N. Circulating tumor cells in the early detection of human cancers[J]. Int. J. Biol. Sci., 2022, 18(8): 3251-3265. |
| [35] | Song Y, Tian T, Shi Y, Liu W L, Zou Y, Khajvand T, Wang S L, Zhu Z, Yang C Y. Enrichment and single-cell analysis of circulating tumor cells[J]. Chem. Sci., 2017, 8(3): 1736-1751. |
| [36] | Akpe V, Kim T H, Brown C L, Cock I E. Circulating tumor cells: a broad perspective[J]. J. R. Soc. Interface, 2020, 17(168): 20200065. |
| [37] | Bigall N C, Ha?rtling T, Klose M, Klose M, Simon P, Eng L M, Eychmüller A. Monodisperse platinum nanospheres with adjustable diameters from 10 to 100 nm: synthesis and distinct optical properties[J]. Nano lett., 2008, 8(12): 4588-4592. |
/
| 〈 |
|
〉 |