

电化学方法诱导强金属-载体相互作用提高氢氧化反应催化剂的CO耐受性
#Equal contributions
收稿日期: 2024-04-26
修回日期: 2024-05-23
录用日期: 2024-06-02
网络出版日期: 2024-05-30
Electrochemical-Method-Induced Strong Metal-Support Interaction in Pt-CNT@SnO2 for CO-Tolerant Hydrogen Oxidation Reaction
Received date: 2024-04-26
Revised date: 2024-05-23
Accepted date: 2024-06-02
Online published: 2024-05-30
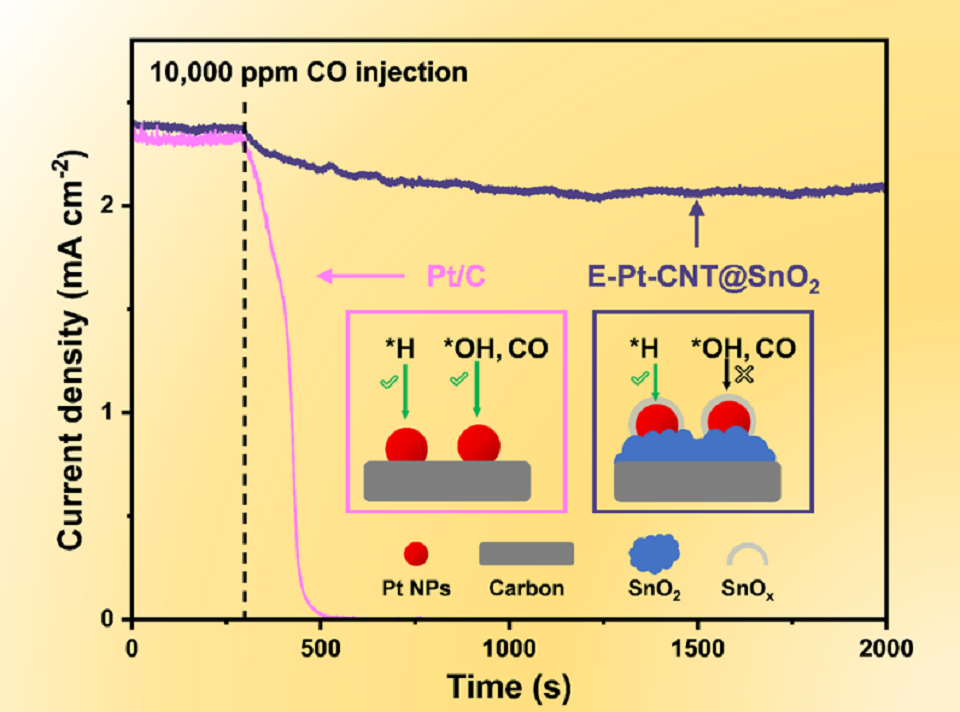
诱导经典强金属-载体相互作用(SMSI)是一种提高负载型金属催化剂性能的有效途径,这指的是载体包覆金属纳米颗粒的结构重构效应。传统的SMSI诱导方式是热还原方法,但这过程中往往伴随着会损害催化活性的金属纳米颗粒的长大过程。为了解决这一问题,本研究开发了一种温和的电化学方法来诱导SMSI,并在核壳结构CNT@SnO2载体上负载的Pt纳米颗粒催化剂中进行了验证。高分辨透射电镜(HRTEM)和电化学测试结果证实了电化学诱导方法成功在Pt纳米颗粒表面构建了SnOx包覆层。这种SnOx包覆层可以保护Pt纳米颗粒在氢氧化反应(HOR)中不受CO杂质的毒害。实验显示,当H2中混入10000 ppm浓度的CO时,E-Pt-CNT@SnO2的HOR电流密度经过2000 s后仍能保持85%,而商用Pt/C在相同条件条件下工作300 s则完全失活。此外,SnOx包覆层与Pt纳米颗粒之间存在电子相互作用,这导致电荷从载体迁移到Pt纳米颗粒上,并在远离界面处聚集。这种电荷转移降低了Pt对H中间体的吸附能,提高了E-Pt-CNT@SnO2的HOR活性,催化剂的交换电流密度为1.55 A·mgPt-1,是商业Pt/C的1.3倍。原位拉曼光谱和理论计算结果表明,电化学诱导SMSI的关键因素是Pt纳米颗粒对Sn-O键强度的减弱。此外,Pt纳米颗粒对载体不同区域的Sn-O键强度的弱化存在差异,其中表面Sn原子与内部O原子之间的键强度弱于Sn原子与表面O原子之间的键强度,这促进了SnOx团簇的形成和迁移。
关键词: 强金属-载体相互作用; 铂纳米颗粒; 氢氧化反应; 负载型催化剂; CO耐受性
李申宙 , 林子杰 , 陈麒安 , 蔡钊 , 李箐 . 电化学方法诱导强金属-载体相互作用提高氢氧化反应催化剂的CO耐受性[J]. 电化学, 2024 , 30(12) : 2404121 . DOI: 10.61558/2993-074X.3474
Inducing the classic strong metal-support interaction (SMSI) is an effective approach to enhance the performance of supported metal catalysts by encapsulating the metal nanoparticles (NPs) with supports. Conventional thermal reduction method for inducing SMSI processes is often accompanied by undesirable structural evolution of metal NPs. In this study, a mild electrochemical method has been developed as a new approach to induce SMSI, using the cable structured core@shell CNT@SnO2 loaded Pt NPs as a proof of concept. The induced SnOx encapsulation layer on the surface of Pt NPs can protect Pt NPs from the poisoned of CO impurity in hydrogen oxidation reaction (HOR), and the HOR current density could still maintain 85% for 2000 s with 10000 ppm CO in H2, while the commercial Pt/C is completely inactivated. In addition, the electrons transfer from SnOx to Pt NPs improved the HOR activity of the E-Pt-CNT@SnO2, achieving the excellent exchange current density of 1.55 A·mgPt-1. In situ Raman spectra and theoretical calculations show that the key to the electrochemical-method-induced SMSI is the formation of defects and the migration of SnOx caused by the electrochemical redox operation, and the weakening the Sn-O bond strength by Pt NPs.

/
| 〈 |
|
〉 |