

基于山梨醇添加剂电解质的可逆锌电化学
收稿日期: 2023-11-29
修回日期: 2024-02-18
录用日期: 2024-02-23
网络出版日期: 2024-02-28
Sorbitol-Electrolyte-Additive Based Reversible Zinc Electrochemistry
Received date: 2023-11-29
Revised date: 2024-02-18
Accepted date: 2024-02-23
Online published: 2024-02-28
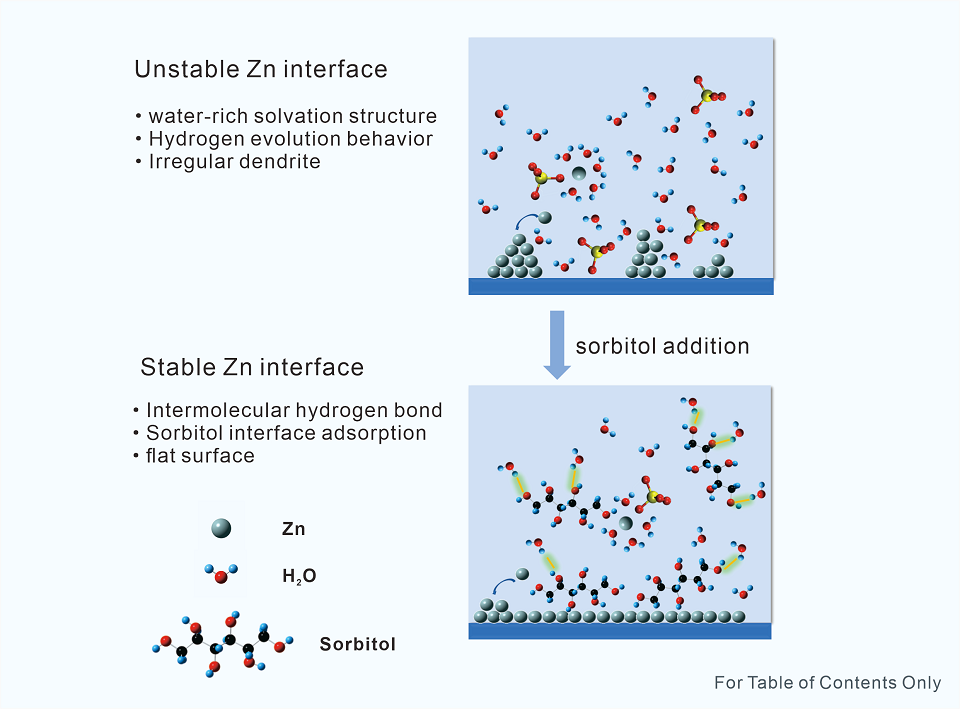
在水系锌离子电池中,锌枝晶、析氢,以及不稳定的锌界面使其表现出低的库仑效率和较差的循环性能。本文选用富含羟基的山梨醇作为电解质添加剂,用于重塑锌的溶剂化结构和调整锌阳极界面化学,有效抑制了水的分解以及锌枝晶的产生。一方面山梨醇与水分子强相互作用可以形成氢键网络,改变Zn2+离子与水分子之间的溶剂化壳层,减少结合水的比例,降低游离水活性;另一方面山梨醇和Zn阳极界面之间的吸附调节了Zn阳极表面结构,减少其与水分子的接触,获得热力学稳定、高度可逆的Zn电化学沉积/溶解。结果表明,含有山梨醇添加剂的Zn/Zn对称电池在1 mA·cm-2和1 mAh·cm-2的条件下实现了长达2000小时的循环寿命,在5 mA·cm-2和5 mAh·cm-2条件下也能超过250小时的循环寿命。添加山梨醇的Zn/Cu不对称电池获得了99.6%的库仑效率,比纯2 mol·L-1 ZnSO4电解质具有更高性能。Zn/PNDA全电池可在1 A·g-1电流密度下稳定放电超过2300次,并且保持了较好的电池容量。本论文通过向传统电解质中添加安全无毒的山梨醇,提升了对锌阳极界面的保护作用以及循环性能,改善了锌的可逆沉积/溶解电化学性能,为高性能水系锌离子电池的探索提供了新思路。
孙琼 , 杜海会 , 孙田将 , 李典涛 , 程敏 , 梁静 , 李海霞 , 陶占良 . 基于山梨醇添加剂电解质的可逆锌电化学[J]. 电化学, 2024 , 30(7) : 2314002 . DOI: 10.61558/2993-074X.3447
The unstable zinc (Zn)/electrolyte interfaces formed by undesired dendrites and parasitic side reactions greatly hinder the development of aqueous zinc ion batteries. Herein, the hydroxy-rich sorbitol was used as an additive to reshape the solvation structure and modulate the interface chemistry. The strong interactions among sorbitol and both water molecules and Zn electrode can reduce the free water activity, optimize the solvation shell of water and Zn2+ ions, and regulate the formation of local water (H2O)-poor environment on the surface of Zn electrode, which effectively inhibit the decomposition of water molecules, and thus, achieve the thermodynamically stable and highly reversible Zn electrochemistry. As a result, the assembled Zn/Zn symmetric cells with the sorbitol additive realized an excellent cycling life of 2000 h at 1 mA·cm-2 and 1 mAh·cm-2, and over 250 h at 5 mA·cm-2 and 5 mAh·cm-2. Moreover, the Zn/Cu asymmetric cells with the sorbitol additive achieved a high Coulombic efficiency of 99.6%, obtaining a better performance than that with a pure 2 mol·L-1 ZnSO4 electrolyte. And the constructed Zn/poly1, 5-naphthalenediamine (PNDA) batteries could be stably discharged for 2300 cycles at 1 A·g-1 with an excellent capacity retention rate. This result indicates that the addition of 1 mol·L-1 non-toxic sorbitol into a conventional ZnSO4 electrolyte can successfully protect the Zn anode interface by improving the electrochemical properties of Zn reversible deposition/decomposition, which greatly promotes its cycle performance, providing a new approach in future development of high performance aqueous Zn ion batteries.

/
| 〈 |
|
〉 |