

二硫化钼和碳纳米管复合物电极用于盐差能转换
收稿日期: 2023-09-16
修回日期: 2023-09-18
录用日期: 2023-09-19
网络出版日期: 2023-09-25
Molybdenum Disulfide and Carbon Nanotubes Composite Electrode for Electrochemical Conversion of Salinity Gradient Energy
Received date: 2023-09-16
Revised date: 2023-09-18
Accepted date: 2023-09-19
Online published: 2023-09-25
海洋占地球水资源总量的97%,地表面积的70%以上。随着化石燃料等不可再生能源的持续消耗与可再生能源的快速发展,人们对海洋资源的利用越来越重视。海洋能包括潮汐能、波浪能、温差能和盐差能等。其中盐差能是海水和淡水相互作用产生的能量,是以化学能形式存在的海洋能,这种能量较多产生在河口处。目前,压力延迟渗透技术、反电渗析技术和电容混合技术是转换盐差能的三种主要技术。本文构建了一种基于电容混合技术的新型盐差电池,使用二硫化钼和多壁碳纳米管复合物电极作为阳极,活性炭作为阴极。将两种不同离子储存机制的材料复合在一起,二硫化钼具有类似石墨烯的层状结构,层间间距约为石墨烯的两倍,是一种可以与钠离子发生插层反应的电池电极材料。多壁碳纳米管具有典型的双电层效应,放电时在其表面吸附钠离子的同时,可以帮助钠离子更快地进入二硫化钼层间,加快离子传输效率和盐差能的转换效率。对该复合材料进行物理和电化学表征,并与活性炭电极组装的盐差电池,测试其盐差能转换能力。浓度响应电压150 mV,经过一个完整的四步循环后,转换能量密度可达6.96 J·g-1。该器件原材料价格较低,并且不使用离子膜,更加环保,为转换盐差能的研究提供了一种新途径。
李家俊 , 张伟彬 , 刘鑫宇 , 杨静蕾 , 尹易 , 杨泽钦 , 马雪婧 . 二硫化钼和碳纳米管复合物电极用于盐差能转换[J]. 电化学, 2024 , 30(6) : 2307121 . DOI: 10.13208/j.electrochem.2307121
The ocean accounts for 97% of the total water resources on earth, covering over 70% of the map's surface area. With the continuous consumption of non-renewable energy sources such as fossil fuels and the rapid development of renewable energy, humans are increasingly paying attention to the utilization of ocean resources. Ocean energy includes tidal energy, wave energy, temperature difference energy, and salinity gradient energy. Salinity gradient energy is the energy generated by the interaction of seawater and fresh water, which is the ocean energy existing in the form of chemical energy. This energy is mostly generated in estuaries. The osmotic pressure generated by mixing water with different salinity can be converted into electrical energy driven by potential differences or ion gradients. Salinity gradient energy, as a new renewable energy source, has received widespread global attention and research in recent years, making rapid progress. The utilization of salinity gradient energy provides a renewable and sustainable alternative to the recent surge in global energy consumption.
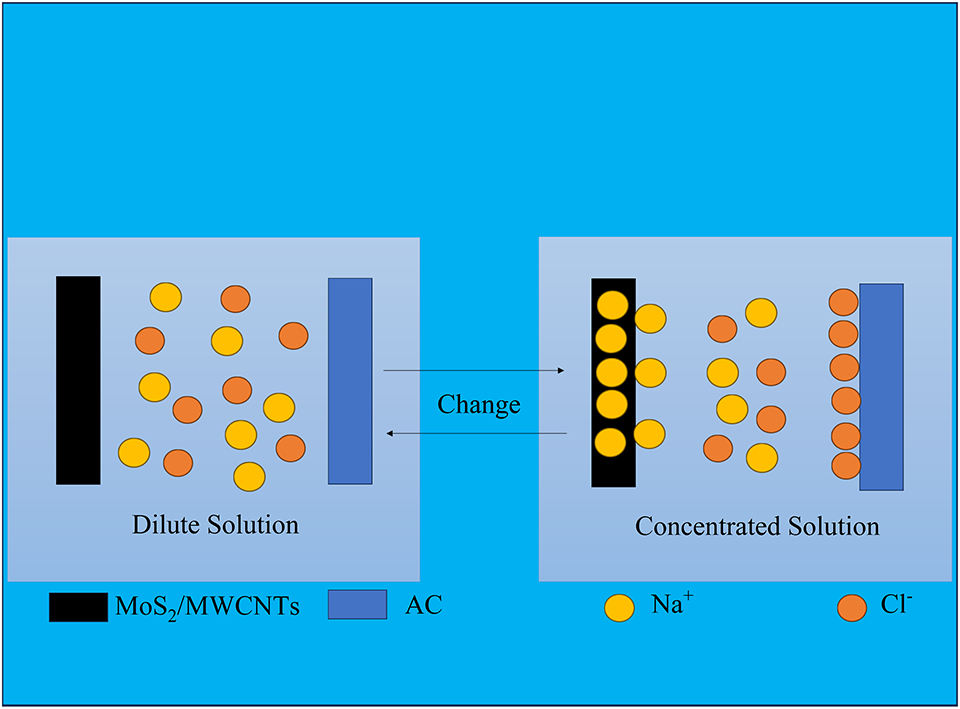
At present, pressure delay osmosis technology, reverse electrodialysis technology and capacitive mixing technology are three main technologies for extracting salinity gradient energy. In this work, we built a new type of salt difference cell based on capacitive mixing technology, using molybdenum disulfide (MoS2) and multiwalled carbon nanotubes (MoS2/MWCNTs) composite electrode as the anode and an activated carbon (AC) as the cathode.
We composited two materials with different ion storage mechanisms together. MoS2 has a layered structure like graphene, with an interlayer spacing of about twice that of graphene. It is a battery electrode material that can undergo intercalation reaction with Na+. MWCNTs have a typical double electric layer effect. When discharging, while adsorbing Na+ on its surface, it can help Na+ enter the interlayer of MoS2 more quickly, accelerating the ion transport efficiency and the extraction efficiency of salt differential energy. We conducted physical and electrochemical characterizations of MoS2/MWCNTs composite material, and tested its salt difference energy extraction ability on a salt difference battery composed of it and AC electrode. We found that the concentration response voltage reached 150 mV, and the energy density of the extracted salt difference energy after a complete four-step cycle reached up to 6.96 J·g-1. The advantages of low raw material price of the device and without using ion membranes make it more environmentally friendly, providing a new approach for the study of extracting salinity gradient energy.

/
| 〈 |
|
〉 |