

用于促进碱性介质中析氢反应动力学的异质结构电催化剂的合理设计
收稿日期: 2023-05-10
修回日期: 2023-08-08
录用日期: 2023-08-22
网络出版日期: 2023-09-09
Rational Design of Heterostructured Nanomaterials for Accelerating Electrocatalytic Hydrogen Evolution Reaction Kinetics in Alkaline Media
Received date: 2023-05-10
Revised date: 2023-08-08
Accepted date: 2023-08-22
Online published: 2023-09-09
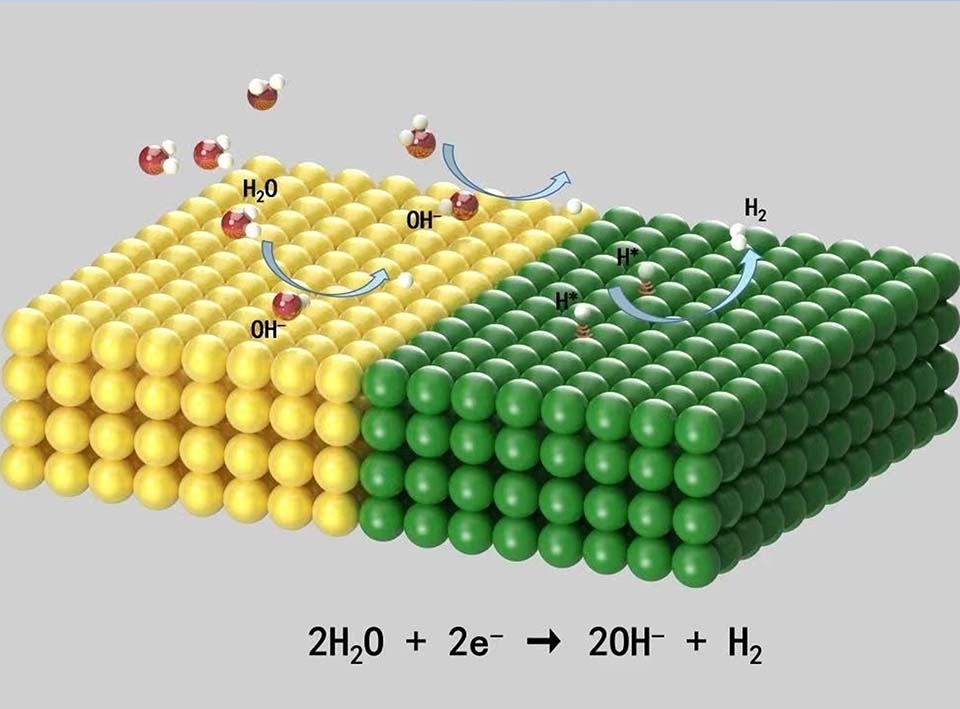
在碱性介质中,由于电极材料的较高的稳定性,电催化析氢反应(HER)具有实现大规模制氢的巨大潜力。然而,即使对于最突出的铂催化剂,HER在碱性介质中的反应动力学也比在酸性介质中慢2-3个数量级,这是由于碱性环境下质子的浓度较低。异质结构催化剂具有多种结构优势,研究表明,构建异质结构电催化剂是促进碱性HER动力学的有效策略。协同效应是异质结构的一个独特特征,这意味着一个功能活性位点作为水解离的促进剂,另一个活性位点则负责适度的氢吸附,从而协同提高HER催化性能。此外,异质结构中的每个构建模块都是可调节的,为构建最佳催化剂提供了更多的灵活性和可能性。同时,由于界面处两个组分之间存在费米能级差,可以合理地调控每个组分的电子结构,从而大幅度提高碱性介质中的HER催化性能。随着对纳米结构的深入理解,人们开发了更先进的设计策略来构建高性能异质结构电催化剂。本文综述了异质结构催化剂在碱性HER方面的最新发展,以及构建界面异质结构以促进碱性HER动力学性能的合理设计原则。我们首先介绍了HER在碱性介质中的基本反应途径,然后详细讨论了促进碱性HER动力学的新兴有效策略,包括协同效应、应变效应、电子相互作用、相工程和结构工程,最后提出了未来面向实际应用的新型异质结构催化剂设计所面临的挑战和研究机遇。
马海斌 , 周晓延 , 李嘉艺 , 程洪飞 , 马吉伟 . 用于促进碱性介质中析氢反应动力学的异质结构电催化剂的合理设计[J]. 电化学, 2024 , 30(1) : 2305101 . DOI: 10.13208/j.electrochem.2305101
Owing to the merits of high energy density, as well as clean and sustainable properties, hydrogen has been deemed to be a prominent alternative energy to traditional fossil fuels. Electrocatalytic hydrogen evolution reaction (HER) has been considered to be mostly promising for achieving green hydrogen production, and has been widely studied in acidic and alkaline solutions. In particular, HER in alkaline media has high potential to achieve large-scale hydrogen production because of the increased durability of electrode materials. However, for the currently most prominent catalyst Pt, its HER kinetics in an alkaline solution is generally 2-3 orders lower than that occurring in an acidic solution because of the low H+ concentration in alkaline electrolytes. Fortunately, construction of heterostructured electrocatalysts has proved to be an efficient strategy for boosting alkaline HER kinetics because of their various structural merits. The synergistic effect is a unique characteristic of heterostructures, which means that one functional active site serves as a promoter for water dissociation and another one takes a charge of moderate hydrogen adsorption, thus synergistically improving HER performance. In addition, each building block of the heterostructures is tunable, providing more flexibility and chances to construct optimal catalysts. Furthermore, due to the presence of Fermi energy difference between the two components at the interface, the electronic structure of each component could possibly be rationally modulated, thus much enhanced HER performance in alkaline electrolyte can be achieved. With a deeper understanding of on nanoscience and rapid development of nanotechnology, more sophisticated alternative designing strategies have been explored for constructing high-performance heterostructured electrocatalysts. This review presents an outline of the latest development of heterostructured catalysts toward alkaline HER and the rational design principles for constructing interfacial heterostructures to accelerate alkaline HER kinetics. The basic reaction pathways of HER in alkaline media are first described, and then emerging efficient strategies to promote alkaline HER kinetics, including synergistic effect, strain effect, electronic interaction, phase engineering, and architecture engineering. Finally, current existing challenges and research opportunities that deserve further investigation are proposed for the consideration of novel heterostructures towards practical applications.

| [1] | Debe M K. Electrocatalyst approaches and challenges for automotive fuel cells[J]. Nature, 2012, 486(7401): 43-51. |
| [2] | Zheng Y, Jiao Y, Jaroniec M, Qiao S Z. Advancing the electrochemistry of the hydrogen-evolution reaction through combining experiment and theory[J]. Angew. Chem. Int. Ed., 2015, 54(1): 52-65. |
| [3] | Yan Y, He T, Zhao B, Qi K, Liu H, Xia B Y. Metal/covalent-organic frameworks-based electrocatalysts for water splitting[J]. J. Mater. Chem. A, 2018, 6(33): 15905-15926. |
| [4] | Pan J, Xu Y Y, Yang H, Dong Z, Liu H, Xia B Y. Advanced architectures and relatives of air electrodes in Zn-air batteries[J]. Adv. Sci., 2018, 5(4): 1700691. |
| [5] | Zhang J Y, Wang H, Tian Y, Yan Y, Xue Q, He T, Liu H, Wang C, Chen Y, Xia B Y. Anodic hydrazine oxidation assists energy-efficient hydrogen evolution over a bifunctional cobalt perselenide nanosheet electrode[J]. Angew. Chem. Int. Ed., 2018, 57(26): 7649-7653. |
| [6] | Miao M, Pan J, He T, Yan Y, Xia B Y, Wang X. Molybdenum carbide-based electrocatalysts for hydrogen evolution reaction[J]. Chemistry - A European Journal, 2017, 23(46): 10947-10961. |
| [7] | Lu F, Zhou M, Zhou Y X, Zeng X H. First-row transition metal based catalysts for the oxygen evolution reaction under alkaline conditions: Basic principles and recent advances[J]. Small, 2017, 13(45): 1701931. |
| [8] | Mahmood N, Yao Y, Zhang J W, Pan L, Zhang X, Zou J J. Electrocatalysts for hydrogen evolution in alkaline electrolytes: Mechanisms, challenges, and prospective solutions[J]. Adv. Sci., 2018, 5(2): 1700464. |
| [9] | Zheng Y, Jiao Y, Vasileff A, Qiao S Z. The hydrogen evolution reaction in alkaline solution: From theory, single crystal models, to practical electrocatalysts[J]. Angew. Chem. Int. Ed., 2018, 57(26): 7568-7579. |
| [10] | Gong M, Wang D Y, Chen C C, Hwang B J, Dai H. A mini review on nickel-based electrocatalysts for alkaline hydrogen evolution reaction[J]. Nano Res., 2016, 9(1): 28-46. |
| [11] | Seh Z W, Kibsgaard J, Dickens C F, Chorkendorff I, N?rskov J K, Jaramillo T F. Combining theory and experiment in electrocatalysis: insights into materials design[J]. Science, 2017, 355(6321): eaad4998. |
| [12] | Subbaraman R, Tripkovic D, Strmcnik D, Chang K C, Uchimura M, Paulikas A P, Stamenkovic V, Markovic N M. Enhancing hydrogen evolution activity in water splitting by tailoring Li+-Ni(OH)2-Pt interfaces[J]. Science, 2011, 334(6060): 1256-1260. |
| [13] | Yang H C, Wang C H, Hu F, Zhang Y J, Lu H, Wang Q B. Atomic-scale pt clusters decorated on porous α-Ni(OH)2 nanowires as highly efficient electrocatalyst for hydrogen evolution reaction[J]. Sci. China Mater., 2017, 60(11): 1121-1128. |
| [14] | Li W B, Song Q Q, Li M, Yuan Y F, Zhang J H, Wang N, Yang Z H, Huang J F, Lu J, Li X F. Chemical heterointerface engineering on hybrid electrode materials for electrochemical energy storage[J]. Small Methods, 2021, 5(8): 2100444. |
| [15] | Shao Q, Wang P T, Huang X Q. Opportunities and challenges of interface engineering in bimetallic nanostructure for enhanced electrocatalysis[J]. Adv. Funct. Mater., 2019, 29(3): 1806419. |
| [16] | Du P, Cao L, Zhang B, Wang C H, Xiao Z M, Zhang J F, Wang D, Ou X. Recent progress on heterostructure materials for next-generation sodium/potassium ion batteries[J]. Renew. Sust. Energ. Rev., 2021, 151: 111640. |
| [17] | Zheng D, Yu L H, Liu W X, Dai X J, Niu X X, Fu W Q, Shi W H, Wu F F, Cao X H. Structural advantages and enhancement strategies of heterostructure water-splitting electrocatalysts[J]. Cell Rep. Phys. Sci., 2021, 2(6): 100443. |
| [18] | Chen P Z, Tong Y, Wu C Z, Xie Y. Surface/interfacial engineering of inorganic low-dimensional electrode materials for electrocatalysis[J]. Acc. Chem. Res., 2018, 51(11): 2857-2866. |
| [19] | Mahmood J, Li F, Jung S M, Okyay M S, Ahmad I, Kim S J, Park N, Jeong H Y, Baek J B. An efficient and pH-universal ruthenium-based catalyst for the hydrogen evolution reaction[J]. Nat. Nanotechnol., 2017, 12(5): 441-446. |
| [20] | Kibsgaard J, Chorkendorff I. Considerations for the scaling-up of water splitting catalysts[J]. Nat. Energy, 2019, 4(6): 430-433. |
| [21] | Zou X, Zhang Y. Noble metal-free hydrogen evolution catalysts for water splitting[J]. Chem. Soc. Rev., 2015, 44(15): 5148-5180. |
| [22] | Jiao Y, Zheng Y, Jaroniec M, Qiao S Z. Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions[J]. Chem. Soc. Rev., 2015, 44(8): 2060-2086. |
| [23] | Zhu J, Hu L, Zhao P, Lee L Y S, Wong K Y. Recent advances in electrocatalytic hydrogen evolution using nanoparticles[J]. Chem. Rev., 2020, 120(2): 851-918. |
| [24] | Zhou H, Yu F, Sun J, He R, Chen S, Chu C W, Ren Z. Highly active catalyst derived from a 3D foam of Fe(PO3)2/Ni2P for extremely efficient water oxidation[J]. PNAS, 2017, 114(22): 5607-5611. |
| [25] | Wu H B, Xia B Y, Yu L, Yu X Y, Lou X W. Porous molybdenum carbide nano-octahedrons synthesized via confined carburization in metal-organic frameworks for efficient hydrogen production[J]. Nat. Commun., 2015, 6(1): 6512. |
| [26] | Yin J, Fan Q H, Li Y X, Cheng F Y, Zhou P P, Xi P X, Sun S H. Ni-C-N nanosheets as catalyst for hydrogen evolution reaction[J]. J. Am. Chem. Soc., 2016, 138(44): 14546-14549. |
| [27] | Ma Y Y, Wu C X, Feng X J, Tan H Q, Yan L K, Liu Y, Kang Z H, Wang E B, Li Y G. Highly efficient hydrogen evolution from seawater by a low-cost and stable CoMoP@C electrocatalyst superior to Pt/C[J]. Energy Environ. Sci., 2017, 10(3): 788-798. |
| [28] | Tan Y W, Wang H, Liu P, Cheng C, Zhu F, Hirata A, Chen M W. 3D nanoporous metal phosphides toward high-efficiency electrochemical hydrogen production[J]. Adv. Mater., 2016, 28(15): 2951-2955. |
| [29] | Hu J, Zhang C X, Jiang L, Lin H, An Y M, Zhou D, Leung M K H, Yang S H. Nanohybridization of MoS2 with layered double hydroxides efficiently synergizes the hydrogen evolution in alkaline media[J]. Joule, 2017, 1(2): 383-393. |
| [30] | Staszak-Jirkovsky J, Malliakas Christos D, Lopes Pietro P, Danilovic N, Kota Subrahmanyam S, Chang K C, Genorio B, Strmcnik D, Stamenkovic Vojislav R, Kanatzidis M G, Markovic N M. Design of active and stable Co-Mo-Sxchalcogels as pH-universal catalysts for the hydrogen evolution reaction[J]. Nat. Mater., 2016, 15(2): 197-203. |
| [31] | Martin-Sabi M, Soriano-López J, Winter R S, Chen J J, Vilà-Nadal L, Long D L, Galán-Mascarós J R, Cronin L. Redox tuning the weakley-type polyoxometalate archetype for the oxygen evolution reaction[J]. Nat. Catal., 2018, 1(3): 208-213. |
| [32] | Liu B, Wang Y, Peng H Q, Yang R, Jiang Z, Zhou X, Lee C S, Zhao H, Zhang W. Iron vacancies induced bifunctionality in ultrathin feroxyhyte nanosheets for overall water splitting[J]. Adv. Mater., 2018, 30(36): 1803144. |
| [33] | Yao R Q, Shi H, Wan W B, Wen Z, Lang X Y, Jiang Q. Flexible Co-Mo-N/Au electrodes with a hierarchical nanoporous architecture as highly efficient electrocatalysts for oxygen evolution reaction[J]. Adv. Mater., 2020, 32(10): 1907214. |
| [34] | Fl?ry N, Ma P, Salamin Y, Emboras A, Taniguchi T, Watanabe K, Leuthold J, Novotny L. Waveguide-integrated van der waals heterostructure photodetector at telecom wavelengths with high speed and high responsivity[J]. Nat. Nanotechnol., 2020, 15(2): 118-124. |
| [35] | Li Y, Zhang J W, Chen Q G, Xia X H, Chen M H. Emerging of heterostructure materials in energy storage: A review[J]. Adv. Mater., 2021, 33(27): 2100855. |
| [36] | Wang H, Tzeng Y K, Ji Y, Li Y, Li J, Zheng X, Yang A, Liu Y, Gong Y, Cai L, Li Y, Zhang X, Chen W, Liu B, Lu H, Melosh N A, Shen Z X, Chan K, Tan T, Chu S, Cui Y. Synergistic enhancement of electrocatalytic CO2 reduction to C2oxygenates at nitrogen-doped nanodiamonds/Cu interface[J]. Nat. Nanotechnol., 2020, 15(2): 131-137. |
| [37] | Du F, Shi L, Zhang Y T, Li T, Wang J L, Wen G H, Alsaedi A, Hayat T, Zhou Y, Zou Z G. Foam-like Co9S8/Ni3S2 heterostructure nanowire arrays for efficient bifunctional overall water-splitting[J]. Appl. Catal., B, 2019, 253: 246-252. |
| [38] | Han X T, Niu Y Y, Yu C, Liu Z B, Huang H W, Huang H L, Li S F, Guo W, Tan X Y, Qiu J S. Ultrafast construction of interfacial sites by wet chemical etching to enhance electrocatalytic oxygen evolution[J]. Nano Energy, 2020, 69: 104367. |
| [39] | An L, Zhang Z, Feng J, Lv F, Li Y, Wang R, Lu M, Gupta R B, Xi P, Zhang S. Heterostructure-promoted oxygen electrocatalysis enables rechargeable zinc-air battery with neutral aqueous electrolyte[J]. J. Am. Chem. Soc., 2018, 140(50): 17624-17631. |
| [40] | Zheng X R, Han X P, Cao Y H, Zhang Y, Nordlund D, Wang J H, Chou S L, Liu H, Li L L, Zhong C, Deng Y D, Hu W B. Identifying dense NiSe2/CoSe2heterointerfaces coupled with surface high-valence bimetallic sites for synergistically enhanced oxygen electrocatalysis[J]. Adv. Mater., 2020, 32(26): 2000607. |
| [41] | Wang P T, Qiao M, Shao Q, Pi Y C, Zhu X, Li Y F, Huang X Q. Phase and structure engineering of copper tin heterostructures for efficient electrochemical carbon dioxide reduction[J]. Nat. Commun., 2018, 9(1): 4933. |
| [42] | Xin Z K, Gao Y J, Gao Y, Song H W, Zhao J, Fan F, Xia A D, Li X B, Tung C H, Wu L Z. Rational design of dot-on-rod nano-heterostructure for photocatalytic CO2 reduction: pivotal role of hole transfer and utilization[J]. Adv. Mater., 2022, 34(3): 2106662. |
| [43] | Mei J, Liao T, Sun Z Q. 2D/2D heterostructures: Rational design for advanced batteries and electrocatalysis[J]. Energy Environ. Mater., 2022, 5(1): 115-132. |
| [44] | Zhou W, Cheng C, Liu J, Tay Y Y, Jiang J, Jia X, Zhang J, Gong H, Hng H H, Yu T, Fan H J. Epitaxial growth of branched α-Fe2O3/SnO2 nano-heterostructures with improved lithium-ion battery performance[J]. Adv. Funct. Mater., 2011, 21(13): 2439-2445. |
| [45] | Sun D D, Liu K H, Hu J P, Zhou J S. Antiblocking heterostructure to accelerate kinetic process for Na-ion storage[J]. Small, 2021, 17(4): 2006374. |
| [46] | Wang T S, Legut D, Fan Y C, Qin J, Li X F, Zhang Q F. Building fast diffusion channel by constructing metal sulfide/metal selenide heterostructures for high-performance sodium ion batteries anode[J]. Nano Lett., 2020, 20(8): 6199-6205. |
| [47] | Wang L, Zhu Y H, Zeng Z H, Lin C, Giroux M, Jiang L, Han Y, Greeley J, Wang C, Jin J. Platinum-nickel hydroxide nanocomposites for electrocatalytic reduction of water[J]. Nano Energy, 2017, 31: 456-461. |
| [48] | Zhang B, Liu J, Wang J S, Ruan Y J, Ji X, Xu K, Chen C, Wan H Z, Miao L, Jiang J J. Interface engineering: The Ni(OH)2/MoS2 heterostructure for highly efficient alkaline hydrogen evolution[J]. Nano Energy, 2017, 37: 74-80. |
| [49] | Sheng W, Myint M, Chen J G, Yan Y. Correlating the hydrogen evolution reaction activity in alkaline electrolytes with the hydrogen binding energy on monometallic surfaces[J]. Energy Environ. Sci., 2013, 6(5): 1509-1512. |
| [50] | Dinh C T, Jain A, De Arquer F P G, De Luna P, Li J, Wang N, Zheng X, Cai J, Gregory B Z, Voznyy O, Zhang B, Liu M, Sinton D, Crumlin E J, Sargent E H. Multi-site electrocatalysts for hydrogen evolution in neutral media by destabilization of water molecules[J]. Nat. Energy, 2019, 4(2): 107-114. |
| [51] | Tao H B, Zhang J, Chen J, Zhang L, Xu Y, Chen J G, Liu B. Revealing energetics of surface oxygen redox from kinetic fingerprint in oxygen electrocatalysis[J]. J. Am. Chem. Soc., 2019, 141(35): 13803-13811. |
| [52] | Mccrum I T, Koper M T M. The role of adsorbed hydroxide in hydrogen evolution reaction kinetics on modified platinum[J]. Nat. Energy, 2020, 5(11): 891-899. |
| [53] | Anantharaj S, Noda S, Jothi V R, Yi S, Driess M, Menezes P W. Strategies and perspectives to catch the missing pieces in energy-efficient hydrogen evolution reaction in alkaline media[J]. Angew. Chem. Int. Ed., 2021, 60(35): 18981-19006. |
| [54] | Chen Y Y, Zhang Y, Zhang X, Tang T, Luo H, Niu S, Dai Z H, Wan L J, Hu J S. Self-templated fabrication of MoNi4/MoO3-x nanorod arrays with dual active components for highly efficient hydrogen evolution[J]. Adv. Mater., 2017, 29(39): 1703311. |
| [55] | Zhang J, Wang T, Liu P, Liao Z Q, Liu S H, Zhuang X D, Chen M W, Zschech E, Feng X L. Efficient hydrogen production on MoNi4 electrocatalysts with fast water dissociation kinetics[J]. Nat. Commun., 2017, 8(1): 15437. |
| [56] | Brown D E, Mahmood M N, Man M C M, Turner A K. Preparation and characterization of low overvoltage transition metal alloy electrocatalysts for hydrogen evolution in alkaline solutions[J]. Electrochim. Acta, 1984, 29(11): 1551-1556. |
| [57] | Mckone J R, Sadtler B F, Werlang C A, Lewis N S, Gray H B. Ni-Mo nanopowders for efficient electrochemical hydrogen evolution[J]. ACS Catal., 2013, 3(2): 166-169. |
| [58] | An Y M, Long X, Ma M, Hu J, Lin H, Zhou D, Xing Z, Huang B L, Yang S H. One-step controllable synthesis of catalytic Ni4Mo/MoOx/Cu nanointerfaces for highly efficient water reduction[J]. Adv. Energy Mater., 2019, 9(41): 1901454. |
| [59] | Wang L, Lin C, Huang D K, Chen J M, Jiang L, Wang M K, Chi L F, Shi L, Jin J. Optimizing the volmer step by single-layer nickel hydroxide nanosheets in hydrogen evolution reaction of platinum[J]. ACS Catal., 2015, 5(6): 3801-3806. |
| [60] | Xu K, Ding H, Zhang M X, Chen M, Hao Z, Zhang L, Wu C Z, Xie Y. Regulating water-reduction kinetics in cobalt phosphide for enhancing her catalytic activity in alkaline solution[J]. Adv. Mater., 2017, 29(28): 1606980. |
| [61] | Zhang J, Wang T, Liu P, Liu S H, Dong R H, Zhuang X D, Chen M W, Feng X L. Engineering water dissociation sites in MoS2 nanosheets for accelerated electrocatalytic hydrogen production[J]. Energy Environ. Sci., 2016, 9(9): 2789-2793. |
| [62] | Weng Z, Liu W, Yin L C, Fang R, Li M, Altman E I, Fan Q, Li F, Cheng H M, Wang H. Metal/oxide interface nanostructures generated by surface segregation for electrocatalysis[J]. Nano Lett., 2015, 15(11): 7704-7710. |
| [63] | Zhang H, Maijenburg A W, Li X, Schweizer S L, Wehrspohn R B. Bifunctional heterostructured transition metal phosphides for efficient electrochemical water splitting[J]. Adv. Funct. Mater., 2020, 30(34): 2003261. |
| [64] | Wei J, Zhou M, Long A, Xue Y, Liao H, Wei C, Xu Z J. Heterostructured electrocatalysts for hydrogen evolution reaction under alkaline conditions[J]. Nano-Micro Lett., 2018, 10(4): 75. |
| [65] | Li J, Li B, Huang H, Yan S, Yuan C Z, Wu N T, Guo D L, Liu X M. Polyvinylpyrrolidone gel based Pt/Ni(OH)2 heterostructures with redistributing charges for enhanced alkaline hydrogen evolution reaction[J]. J. Mater. Chem. A, 2021, 9(47): 27061-27071. |
| [66] | Wang C, Qi L M. Heterostructured inter-doped ruthenium-cobalt oxide hollow nanosheet arrays for highly efficient overall water splitting[J]. Angew. Chem. Int. Ed., 2020, 59(39): 17219-17224. |
| [67] | Zhou A, Guo W J, Wang Y Q, Zhang J T. The rapid preparation of efficient mofeco-based bifunctional electrocatalysts via joule heating for overall water splitting[J]. J. Electrochem., 2022, 28(9): 2214007. |
| [68] | Xu Q C, Zhang J H, Zhang H X, Zhang L Y, Chen L, Hu Y J, Jiang H, Li C Z. Atomic heterointerface engineering overcomes the activity limitation of electrocatalysts and promises highly-efficient alkaline water splitting[J]. Energy Environ. Sci., 2021, 14(10): 5228-5259. |
| [69] | Hammer B, Norskov J K. Why gold is the noblest of all the metals[J]. Nature, 1995, 376(6537): 238-240. |
| [70] | Strasser P, Koh S, Anniyev T, Greeley J, More K, Yu C, Liu Z, Kaya S, Nordlund D, Ogasawara H, Toney M F, Nilsson A. Lattice-strain control of the activity in dealloyed core-shell fuel cell catalysts[J]. Nat. Chem., 2010, 2(6): 454-460. |
| [71] | Karlberg G S. Adsorption trends for water, hydroxyl, oxygen, and hydrogen on transition-metal and platinum-skin surfaces[J]. Phys. Rev. B, 2006, 74(15): 153414. |
| [72] | Skúlason E, Tripkovic V, Bj?rketun M E, Gudmundsdóttir S, Karlberg G, Rossmeisl J, Bligaard T, Jónsson H, N?rskov J K. Modeling the electrochemical hydrogen oxidation and evolution reactions on the basis of density functional theory calculations[J]. J. Phys. Chem. C, 2010, 114(42): 18182-18197. |
| [73] | Zhao G Q, Jiang Y Z, Dou S X, Sun W P, Pan H G. Interface engineering of heterostructured electrocatalysts towards efficient alkaline hydrogen electrocatalysis[J]. Sci. Bull., 2021, 66(1): 85-96. |
| [74] | Jiang Z L, Song S J, Zheng X B, Liang X, Li Z X, Gu H F, Li Z, Wang Y, Liu S H, Chen W X, Wang D S, Li Y D. Lattice strain and schottky junction dual regulation boosts ultrafine ruthenium nanoparticles anchored on a n-modified carbon catalyst for H2 production[J]. J. Am. Chem. Soc., 2022, 144(42): 19619-19626. |
| [75] | Norsko J K. Chemisorption on metal surfaces[J]. Rep. Prog. Phys., 1990, 53(10): 1253. |
| [76] | N?rskov J K. Electronic factors in catalysis[J]. Prog. Surf. Sci., 1991, 38(2): 103-144. |
| [77] | Xia Z H, Guo S J. Strain engineering of metal-based nanomaterials for energy electrocatalysis[J]. Chem. Soc. Rev., 2019, 48(12): 3265-3278. |
| [78] | Kim J, Kim H, Lee W J, Ruqia B, Baik H, Oh H S, Paek S M, Lim H K, Choi C H, Choi S I. Theoretical and experimental understanding of hydrogen evolution reaction kinetics in alkaline electrolytes with Pt-based core-shell nanocrystals[J]. J. Am. Chem. Soc., 2019, 141(45): 18256-18263. |
| [79] | Zong Z, Xu K, Li D L, Tang Z H, He W, Liu Z, Wang X F, Tian Y. Peptide templated Au@Pd core-shell structures as efficient bi-functional electrocatalysts for both oxygen reduction and hydrogen evolution reactions[J]. J. Catal., 2018, 361: 168-176. |
| [80] | Xiao Z C, Li Z, Jing Y H, Li T, Jiang D Y, Duan Y X, Ye Q X, Zhou L X, Chen A R, Cai J M. Compressive strain induced superior her performance of nickel in alkaline solution[J]. Phys. Chem. Chem. Phys., 2022, 24(45): 27923-27929. |
| [81] | Liang Q M, Wang X, Wan X W, Lin L X, Geng B J, Tian Z Q, Yang Y. Opportunities and challenges of strain engineering for advanced electrocatalyst design[J]. Nano Res., 2023, 16(7): 8655-8669. |
| [82] | Luo M C, Guo S J. Strain-controlled electrocatalysis on multi-metallic nanomaterials[J]. Nat. Rev. Mater., 2017, 2(11): 17059. |
| [83] | Du X C, Huang J W, Zhang J J, Yan Y C, Wu C Y, Hu Y, Yan C Y, Lei T Y, Chen W, Fan C, Xiong J. Modulating electronic structures of inorganic nanomaterials for efficient electrocatalytic water splitting[J]. Angew. Chem. Int. Ed., 2019, 58(14): 4484-4502. |
| [84] | Tang Y, Dong L, Wu H B, Yu X Y. Tungstate-modulated Ni/Ni(OH)2 interface for efficient hydrogen evolution reaction in neutral media[J]. J. Mater. Chem. A, 2021, 9(3): 1456-1462. |
| [85] | Wang H X, Fu W W, Yang X H, Huang Z Y, Li J, Zhang H J, Wang Y. Recent advancements in heterostructured interface engineering for hydrogen evolution reaction electrocatalysis[J]. J. Mater. Chem. A, 2020, 8(15): 6926-6956. |
| [86] | Greeley J, N?rskov J K, Kibler L A, El-Aziz A M, Kolb D M. Hydrogen evolution over bimetallic systems: Understanding the trends[J]. ChemPhysChem, 2006, 7(5): 1032-1035. |
| [87] | Zhang L Y, Hu M H, Li H, Cao B, Jing P, Liu B C, Gao R, Zhang J, Liu B. Boosting hydrogen evolution reaction via electronic coupling of cerium phosphate with molybdenum phosphide nanobelts[J]. Small, 2021, 17(40): 2102413. |
| [88] | Ji L, Wei Y, Wu P, Xu M, Wang T, Wang S, Liang Q, Meyer T J, Chen Z. Heterointerface engineering of Ni2P-Co2P nanoframes for efficient water splitting[J]. Chem. Mater., 2021, 33(23): 9165-9173. |
| [89] | Xu D, Zhang S N, Chen J S, Li X H. Design of the synergistic rectifying interfaces in Mott-Schottky catalysts[J]. Chem. Rev., 2023, 123(1): 1-30. |
| [90] | Wang N, Ning S L, Yu X L, Chen D, Li Z L, Xu J C, Meng H, Zhao D K, Li L G, Liu Q M, Lu B Z, Chen S W. Graphene composites with Ru-RuO2 heterostructures: highly efficient Mott-Schottky-type electrocatalysts for pH-universal water splitting and flexible zinc-air batteries[J]. Appl. Catal., B, 2022, 302: 120838. |
| [91] | Feng J X, Wu J Q, Tong Y X, Li G R. Efficient hydrogen evolution on Cu nanodots-decorated Ni3S2 nanotubes by optimizing atomic hydrogen adsorption and desorption[J]. J. Am. Chem. Soc., 2018, 140(2): 610-617. |
| [92] | Hu Z, Liu Q, Chou S L, Dou S X. Two-dimensional material-based heterostructures for rechargeable batteries[J]. Cell Rep. Phys. Sci., 2021, 2(1): 100286. |
| [93] | Cote L J, Kim J, Tung V C, Luo J, Kim F, Huang J. Graphene oxide as surfactant sheets[J]. Pure Appl. Chem., 2010, 83(1): 95-110. |
| [94] | Backes C, Smith R J, Mcevoy N, Berner N C, Mccloskey D, Nerl H C, O’neill A, King P J, Higgins T, Hanlon D, Scheuschner N, Maultzsch J, Houben L, Duesberg G S, Donegan J F, Nicolosi V, Coleman J N. Edge and confinement effects allow in situ measurement of size and thickness of liquid-exfoliated nanosheets[J]. Nat. Commun., 2014, 5(1): 4576. |
| [95] | Backes C, Szyd?owska B M, Harvey A, Yuan S, Vega-Mayoral V, Davies B R, Zhao P-L, Hanlon D, Santos E J G, Katsnelson M I, Blau W J, Gadermaier C, Coleman J N. Production of highly monolayer enriched dispersions of liquid-exfoliated nanosheets by liquid cascade centrifugation[J]. ACS Nano, 2016, 10(1): 1589-1601. |
| [96] | Wang L N, Hu P, Long Y, Liu Z, He X X. Recent advances in ternary two-dimensional materials: synthesis, properties and applications[J]. J. Mater. Chem. A, 2017, 5(44): 22855-22876. |
| [97] | Liu W X, Yin R L, Xu X L, Zhang L, Shi W H, Cao X H. Structural engineering of low-dimensional metal-organic frameworks: Synthesis, properties, and applications[J]. Adv. Sci., 2019, 6(12): 1802373. |
| [98] | Geng D, Yang H Y. Recent advances in growth of novel 2D materials: beyond graphene and transition metal dichalcogenides[J]. Adv. Mater., 2018, 30(45): 1800865. |
| [99] | Tan C, Cao X, Wu X J, He Q, Yang J, Zhang X, Chen J, Zhao W, Han S, Nam G H, Sindoro M, Zhang H. Recent advances in ultrathin two-dimensional nanomaterials[J]. Chem. Rev., 2017, 117(9): 6225-6331. |
| [100] | Yuan D, Dou Y, He C T, Yu L, Xu L, Adekoya D, Xia Q, Ma J, Dou S X, Zhang S. Sulfur doping optimized intermediate energetics of FeCoOOH for enhanced oxygen evolution catalytic activity[J]. Cell Rep. Phys. Sci., 2021, 2(2): 100331. |
| [101] | Tang C, Zhong L, Zhang B, Wang H F, Zhang Q. 3D mesoporous van der waals heterostructures for trifunctional energy electrocatalysis[J]. Adv. Mater., 2018, 30(5): 1705110. |
| [102] | Muravev V, Parastaev A, Van Den Bosch Y, Ligt B, Claes N, Bals S, Kosinov N, Hensen E J M. Size of cerium dioxide support nanocrystals dictates reactivity of highly dispersed palladium catalysts[J]. Science, 2023, 380(6650): 1174-1179. |
| [103] | Subbaraman R, Tripkovic D, Chang K C, Strmcnik D, Paulikas A P, Hirunsit P, Chan M, Greeley J, Stamenkovic V, Markovic N M. Trends in activity for the water electrolyser reactions on 3D M(Ni,Co,Fe,Mn) hydr(oxy)oxide catalysts[J]. Nat. Mater., 2012, 11(6): 550-557. |
| [104] | Zhu Z, Yin H, He C T, Al-Mamun M, Liu P, Jiang L, Zhao Y, Wang Y, Yang H G, Tang Z, Wang D, Chen X M, Zhao H. Ultrathin transition metal dichalcogenide/3d metal hydroxide hybridized nanosheets to enhance hydrogen evolution activity[J]. Adv. Mater., 2018, 30(28): 1801171. |
| [105] | Chen X Y, Wan J W, Wang J, Zhang Q H, Gu L, Zheng L R, Wang N, Yu R B. Atomically dispersed ruthenium on nickel hydroxide ultrathin nanoribbons for highly efficient hydrogen evolution reaction in alkaline media[J]. Adv. Mater., 2021, 33(44): 2104764. |
| [106] | Yu X, Zhao J, Zheng L R, Tong Y, Zhang M, Xu G, Li C, Ma J, Shi G. Hydrogen evolution reaction in alkaline media: alpha- or beta-nickel hydroxide on the surface of platinum?[J]. ACS Energy Lett., 2018, 3(1): 237-244. |
| [107] | Zheng Y, Jiao Y, Zhu Y, Li L H, Han Y, Chen Y, Jaroniec M, Qiao S Z. High electrocatalytic hydrogen evolution activity of an anomalous ruthenium catalyst[J]. J. Am. Chem. Soc., 2016, 138(49): 16174-16181. |
| [108] | Jin Y C, Zhang M X, Song L, Zhang M D. Research advances in amorphous-crystalline heterostructures toward efficient electrochemical applications[J]. Small, 2023, 19(10): 2206081. |
| [109] | Liu S Q, Wen H R, Ying G, Zhu Y W, Fu X Z, Sun R, Wong C P. Amorphous Ni(OH)2 encounter with crystalline CuS in hollow spheres: A mesoporous nano-shelled heterostructure for hydrogen evolution electrocatalysis[J]. Nano Energy, 2018, 44: 7-14. |
| [110] | Yang M Y, Zhao M X, Yuan J, Luo J X, Zhang J J, Lu Z G, Chen D Z, Fu X Z, Wang L, Liu C. Oxygen vacancies and interface engineering on amorphous/crystalline CrOx-Ni3N heterostructures toward high-durability and kinetically accelerated water splitting[J]. Small, 2022, 18(14): 2106554. |
| [111] | Dong Z H, Lin F, Yao Y H, Jiao L F. Crystalline Ni(OH)2/amorphous NiMoOxmixed-catalyst with Pt-like performance for hydrogen production[J]. Adv. Energy Mater., 2019, 9(46): 1902703. |
| [112] | Huang S C, Meng Y Y, Cao Y F, Yao F, He Z J, Wang X X, Pan H, Wu M M. Amorphous NiWO4 nanoparticles boosting the alkaline hydrogen evolution performance of Ni3S2 electrocatalysts[J]. Appl. Catal., B, 2020, 274: 119120. |
| [113] | Cao D, Wang J Y, Xu H X, Cheng D J. Growth of highly active amorphous RuCu nanosheets on Cu nanotubes for the hydrogen evolution reaction in wide pH values[J]. Small, 2020, 16(37): 2000924. |
| [114] | Kuang M, Zhang J, Liu D, Tan H, Dinh K N, Yang L, Ren H, Huang W, Fang W, Yao J, Hao X, Xu J, Liu C, Song L, Liu B, Yan Q. Amorphous/crystalline heterostructured cobalt-vanadium-iron (oxy)hydroxides for highly efficient oxygen evolution reaction[J]. Adv. Energy Mater., 2020, 10(43): 2002215. |
| [115] | Chang L, Sun Z, Hu Y H. 1T phase transition metal dichalcogenides for hydrogen evolution reaction[J]. Electrochem. Energy Rev., 2021, 4(2): 194-218. |
| [116] | Zhang T, Wu X X, Fan Y F, Shan C F, Wang B K, Xu H J, Tang Y. Hollow CeOx/CoP heterostructures using two-dimensional Co-MOF as template for efficient and stable electrocatalytic water splitting[J]. ChemNanoMat, 2020, 6(7): 1119-1126. |
| [117] | Guo Y N, Tang J, Qian H Y, Wang Z L, Yamauchi Y. One-pot synthesis of zeolitic imidazolate framework 67-derived hollow Co3S4@MoS2 heterostructures as efficient bifunctional catalysts[J]. Chem. Mater., 2017, 29(13): 5566-5573. |
| [118] | Deng K, Ren T L, Xu Y, Liu S L, Dai Z C, Wang Z Q, Li X N, Wang L, Wang H J. Crystalline core-amorphous shell heterostructures: epitaxial assembly of NiB nanosheets onto PtPd mesoporous hollow nanopolyhedra for enhanced hydrogen evolution electrocatalysis[J]. J. Mater. Chem. A, 2020, 8(18): 8927-8933. |
/
| 〈 |
|
〉 |