

面向高性能锂-硫二次电池应用的非对称电极-电解质界面
收稿日期: 2023-04-10
修回日期: 2023-06-11
录用日期: 2023-06-29
网络出版日期: 2023-06-30
Asymmetric Electrode-Electrolyte Interfaces for High-Performance Rechargeable Lithium-Sulfur Batteries
Received date: 2023-04-10
Revised date: 2023-06-11
Accepted date: 2023-06-29
Online published: 2023-06-30
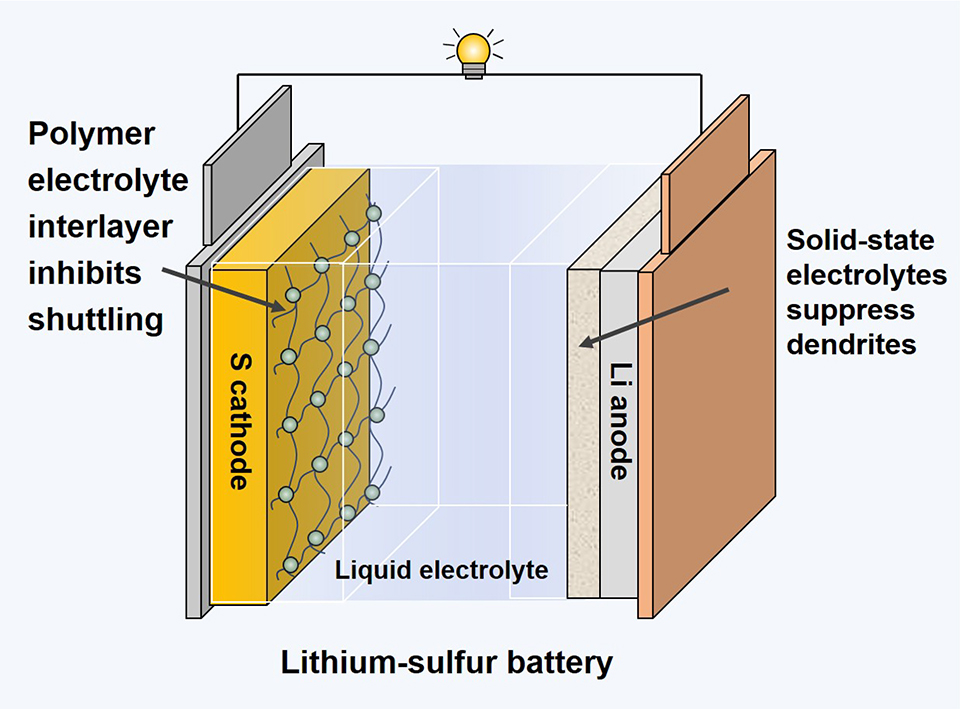
锂-硫电池具有高的理论电芯比能量和低成本,是极具应用前景的下一代电化学储能技术,已被广泛研究。实用化锂-硫电池技术目前面临的挑战主要包括正极侧电活性硫物种在充放电过程中的不可逆损失,负极侧枝晶形核生长,以及因活性硫迁移至负极而导致的界面副反应,上述问题会导致电池工况条件下性能迅速衰退,引发电池失效和安全问题。本工作中,我们提出通过设计非对称的电极-电解质界面稳定锂-硫电池正负极电化学,协同促进电极/电解质体相和界面电荷输运,从而延长电池循环寿命,显著提升电化学性能。本文所讨论的策略有望指导电池界面理性设计,助力实现高性能的锂-硫电池。
丑佳 , 王雅慧 , 王文鹏 , 辛森 , 郭玉国 . 面向高性能锂-硫二次电池应用的非对称电极-电解质界面[J]. 电化学, 2023 , 29(9) : 2217009 . DOI: 10.13208/j.electrochem.2217009
With a high cell-level specific energy and a low cost, lithium-sulfur (Li-S) battery has been intensively studied as one of the most promising candidates for competing the next-generation energy storage campaign. Currently, the practical use of Li-S battery is hindered by the rapidly declined storage performance during battery operation, as caused by irreversible loss of electroactive sulfide species at the cathode, dendrite formation at the anode and parasitic reactions at the electrode-electrolyte interface due to unfavorable cathode-anode crosstalk. In this perspective, we propose to stabilize the Li-S electrochemistry, and improve the storage performance of battery by designing asymmetric electrode-electrolyte interfaces that helps to simultaneously address the differentiated issues at both electrodes and facilitate charge transfer in the electrode/electrolyte and across the interfaces. The strategies discussed would shed lights on reasonable design of battery interfaces towards realization of high-performance Li-S batteries.

/
| 〈 |
|
〉 |