

单分散Cu-TCPP/Cu2O杂化微球:一种具有优异电还原CO2产C2性能的级联电催化剂
收稿日期: 2023-04-07
修回日期: 2023-05-15
录用日期: 2023-06-07
网络出版日期: 2023-06-14
Monodispersed Cu-TCPP/Cu2O Hybrid Microspheres: A Superior Cascade Electrocatalyst toward CO2 Reduction to C2 Products
Received date: 2023-04-07
Revised date: 2023-05-15
Accepted date: 2023-06-07
Online published: 2023-06-14
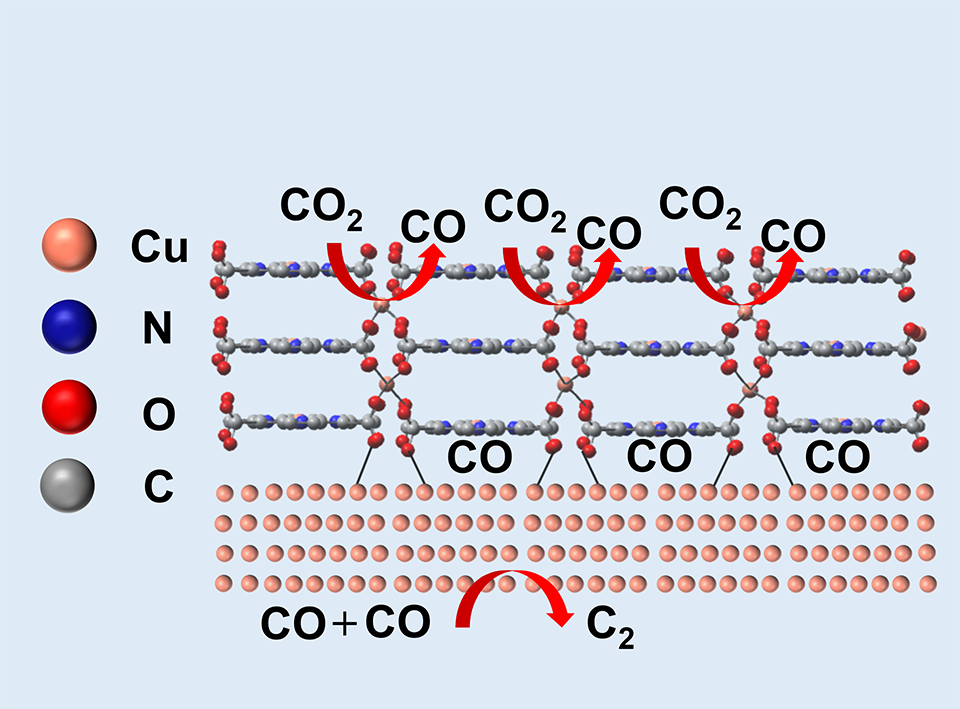
高效电还原CO2(ECR)为有价值的多碳产物是解决CO2排放问题的有效解决方案。基于卟啉的金属有机框架(MOFs)具有多孔结构和有序的活性位点,有望提高ECR生成多碳产物的选择性。本文制备了由铜-四(4-羧基)卟啉(Cu-TCPP)和Cu2O组成的有机/无机杂化Cu-TCPP@Cu2O电催化剂,其中TCPP在调控形貌方面起着重要作用。ECR过程中原位形成的Cu与Cu-TCPP(Cu-TCPP@Cu)结合可以抑制析氢,富集CO中间体,促进C-C偶联生成C2产物。多孔碳(PC)负载的Cu-TCPP@Cu在PC上被还原为Cu纳米簇,同时对C2产物具有较高的ECR活性和选择性。催化剂在-1.0 V时(相对于可逆氢电极),C2产物法拉第效率为62.3%,部分电流密度为83.4 mA·cm-2,是纯Cu2O和TCPP的7.6倍和13.1倍。本论文研究了催化剂形貌和杂化结构如何提高ECR生C2产物的选择性,为高性能ECR催化剂的设计提供了新思路。
关键词: 有机/无机杂化电催化剂; 四(4-羧基)卟啉; 氧化亚铜; 级联电催化剂
万紫轩 , Aidar Kuchkaev , Dmitry Yakhvarov , 康雄武 . 单分散Cu-TCPP/Cu2O杂化微球:一种具有优异电还原CO2产C2性能的级联电催化剂[J]. 电化学, 2024 , 30(1) : 2303271 . DOI: 10.13208/j.electrochem.2303271
The electrochemical conversion of carbon dioxide (CO2) into valuable chemicals is a feasible way to mitigate the negative impacts of overmuch CO2 emissions. Porphyrin-based metal organic frameworks (MOFs) are expected to be used for selective and efficient electrochemical CO2 reduction (ECR) with porous structure and ordered active sites. Herein, we report the synthesis of a monodispersed and spherical organic/inorganic hybrid Cu-TCPP@Cu2O electrocatalyst composed of Cu-TCPP (TCPP=tetrakis (4-carboxyphenyl) porphyrin) and Cu2O, where TCPP plays significant roles in regulating the morphology. In-situ formed Cu during ECR process in combination with Cu-TCPP (Cu-TCPP@Cu) can suppress hydrogen evolution, enrich CO intermediate and promote C-C coupling toward C2 products. The Cu-TCPP@Cu supported on porous carbon (PC) showed ultrafine Cu nanoclusters on PC, and displayed high ECR activity and selectivity toward C2 products, with a C2 faradaic efficiency of 62.3% at -1.0 V versus the reversible hydrogen electrode and a C2 partial current density of 83.4 mA·cm-2, which is 7.6 times and 13.1 times those of pure Cu2O and TCPP, respectively. The morphology and hybrid structure of the catalyst were studied to improve the selectivity of ECR to produce C2 products, which provides a new idea for the design of high-performance ECR catalyst.

/
| 〈 |
|
〉 |