

电极过程动力学反应速率常数测量的若干方法
收稿日期: 2023-04-10
修回日期: 2023-06-27
录用日期: 2023-09-25
网络出版日期: 2023-06-14
基金资助
国家自然科学基金(22202166);国家自然科学基金(21827802);国家自然科学基金(22132003);国家自然科学基金(22021001);中央高校基本科研业务费专项资金(20720230076)
Measurements of Rate Constant for Electrode Reactions
Received date: 2023-04-10
Revised date: 2023-06-27
Accepted date: 2023-09-25
Online published: 2023-06-14
韩联欢 , 郭佳瑶 , 崔苗苗 . 电极过程动力学反应速率常数测量的若干方法[J]. 电化学, 2024 , 30(2) : 2303241 . DOI: 10.13208/j.electrochem.2303241
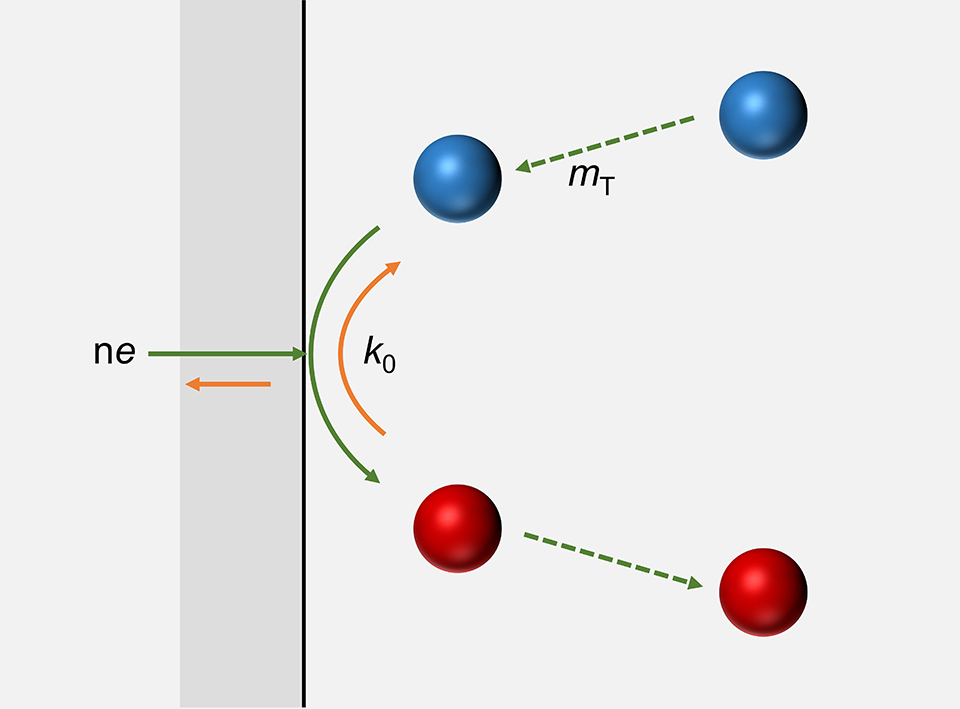
Standard electron-transfer rate constant is one of the intrinsic properties for an electrochemical reaction, which is significant in the study of electrode kinetics. It is a key criterion for one to clarify the mechanism and pathway of a specific electrochemical reaction, and to screening and design the electrocatalysts and battery materials. Herein, we will introduce the measuring methods of rate constant for electrode reactions, including polarization curve, rotating disk electrode, ultramicroelectrode, scanning electrochemical microscopy, electrochemical impedance spectroscopy, current step, potential step and cyclic voltammetry, etc., to provide a guide to investigate electrode kinetics for graduate students and researchers in the related fields.
| [1] | Bard A J, Faulkner L R, White H S. Electrochemical methods: Fundamentals and applications[M]. 3rd. New York: Wiley, 2022. |
| [2] | Cha Q X. Kinetics of electrode process[M]. China: Science Press, 2015. |
| [3] | Zhang Z X. Ultramicroelectrode electrochemistry[M]. China: Science Press, 1998. |
| [4] | Mirkin M V, Bard A J. Simple analysis of quasi-reversible steady-state voltammograms[J]. Anal. Chem., 1992, 64(19): 2293-3302. |
| [5] | Bard A J, Fan F R F; Kwak J, Lev O. Scanning electrochemical microscopy. Introduction and principles[J]. Anal. Chem., 1989, 61(2): 132-138. |
| [6] | Bard A J, Mirkin M V. Scanning electrochemical microscopy[M]. 3rd. New York: CRC Press, 2022. |
| [7] | Sun S G. Fundamentals and methodologies of electrochemical measurement[M]. Xiamen: Xiamen University Press, 2021. |
| [8] | Wipf D O, Bard A J. Scanning electrochemical microscopy X. high resolution imaging of active sites on an electrode surface[J]. J. Electrochem. Soc., 1991, 138: L4. |
| [9] | Zhan D P, Han L H, Zhang J, Shi K, Zhou J Z, Tian Z W, Tian Z Q. Confined chemical etching for electrochemical machining with nanoscale accuracy[J]. Acc. Chem. Res., 2016, 49(11): 2596-3604. |
| [10] | Huang Q A, Park S M. Unified model for transient faradaic impedance spectroscopy: Theory and prediction[J]. J. Phys. Chem. C, 2012, 116(32): 16939-16950. |
| [11] | Cao C N, Zhang J Q. Introduction to electrochemical impedance spectroscopy[M]. China: Science Press, 2002. |
| [12] | Nicholson R S. Theory and application of cyclic voltammetry for measurement of electrode reaction kinetics[J]. Anal. Chem., 1965, 37(11): 1351-1355. |
/
| 〈 |
|
〉 |