

碱性电解槽三维两相CFD模拟研究
收稿日期: 2022-07-08
修回日期: 2023-02-15
录用日期: 2023-02-21
网络出版日期: 2023-02-27
Three-Dimensional Two-Phase CFD Simulation of Alkaline Electrolyzers
Received date: 2022-07-08
Revised date: 2023-02-15
Accepted date: 2023-02-21
Online published: 2023-02-27
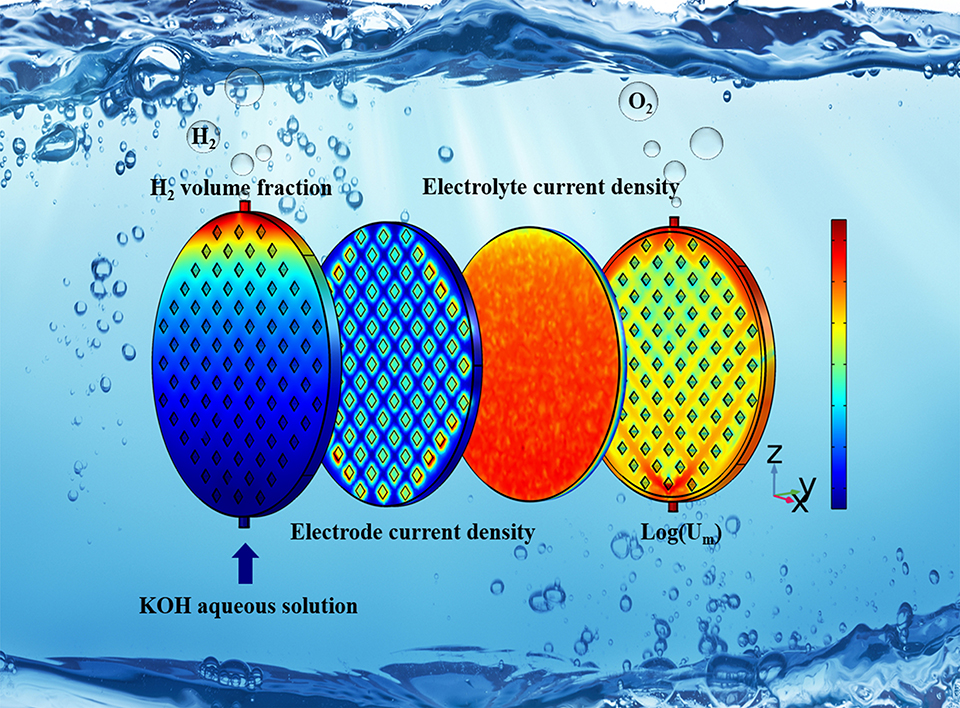
电解槽的结构和运行参数对碱性水电解的性能起着重要作用。针对工业碱性水电解槽紧凑的装配结构,特别是在电流密度大于5000 A·m-2时,本文首次建立了耦合电场和欧拉-欧拉k-ε湍流流场的三维数值模型,以准确模拟碱性水电解槽的性能。将模拟结果与实验数据进行比较,验证了模型的准确性。通过电解槽内部电场和流场特性的反馈,确定了适合的浓度、流量的操作条件和流道结构的优化设计方法。适当增加电解液浓度和流速有利于降低槽电压。KOH水溶液的最佳浓度和流速分别为6.0 - 8.0 mol·L-1和30.0 - 45.0 mL·min-1。随着电极与隔膜距离的增加,欧姆过电压显著增加;流道高度和双极板上导流柱的排列方式对电压的影响微弱,但三角形排列的导流柱和流道高度的增加有利于提高流体的分布均匀度,适当增加导流柱之间的距离有利于降低槽电压。多流体出入口电解槽有利于产生更均匀的流体分布,流道高度对多出入口电解槽同样影响不大。宽导流柱间距的多流体出入口电解槽G-2.5-T-0-5-3,配合高流量,既能降低槽电压,又能提高电解质在电极面的法向流速,使电解槽发挥最佳性能。本工作对碱性水电解高效电解槽的放大设计和优化具有一定指导意义。
高玲玉 , 杨琳 , 王晨辉 , 单桂轩 , 霍欣怡 , 张梦飞 , 李韡 , 张金利 . 碱性电解槽三维两相CFD模拟研究[J]. 电化学, 2023 , 29(9) : 2207081 . DOI: 10.13208/j.electrochem.2207081
The structural and operation parameters of the electrolyzer play important roles in the efficiency of alkaline water electrolysis. In this article, a three-dimensional numerical model coupled with the electric field and the Euler-Eulerian k-ε turbulence flow field was first established to simulate accurately the performance of alkaline electrolyzers, based on a compact assembly structure of the industrial alkaline water electrolyzers, especially at current densities higher than 5000 A·m-2. The simulation results are compared with the experimental data to verify the accuracy of the model. Suitable operating conditions for concentration, flow rate and the optimal design method of the flow channel structure are obtained from the feedback of the electric and flow fields characteristics inside the electrolyzers. Properly increasing the electrolyte concentration and flow rate facilitates the reduction of cell voltage. The optimum concentration and flow rate of potassium hydroxide aqueous solution are evaluated to be 6.0-8.0 mol·L-1 and 30.0-45.0 mL·min-1, respectively. With the increase of the gap between electrode and membrane, the ohmic overpotential increases significantly. The triangular arrangement of conductive columns on the bipolar plate and the increase of the channel height are beneficial to improve the distribution uniformity of the fluid, while the channel height and the arrangement of the conductive columns have little effect on the voltage. Appropriately increasing the spacing between the conductive columns facilitates to reduce the voltage. Multiple outlets and inlets structure is conducive to produce a more uniform fluid distribution. The channel height has little effect on the multiple outlets and inlets electrolyzer. The multiple outlets and inlets electrolyzer G-2.5-T-0-5-3 with wide spacing of conductive columns combined with high flow rate not only can reduce the cell voltage, but also enhance the normal flow rate of the electrolyte on the electrode surface, allowing the best performance of the electrolyzer. This work provides useful guidance on the scale-up design and optimization of highly efficient electrolyzer for alkaline water electrolysis.

/
| 〈 |
|
〉 |