

锂硫电池用高度环化硫化聚丙烯腈的制备
收稿日期: 2022-09-15
修回日期: 2022-10-06
网络出版日期: 2022-11-14
Preparation of Highly-Cyclized Sulfurized Polyacrylonitrile for Lithium-Sulfur Batteries
#These authors contributed equally to this work and should be considered as co-first authors.
Received date: 2022-09-15
Revised date: 2022-10-06
Online published: 2022-11-14
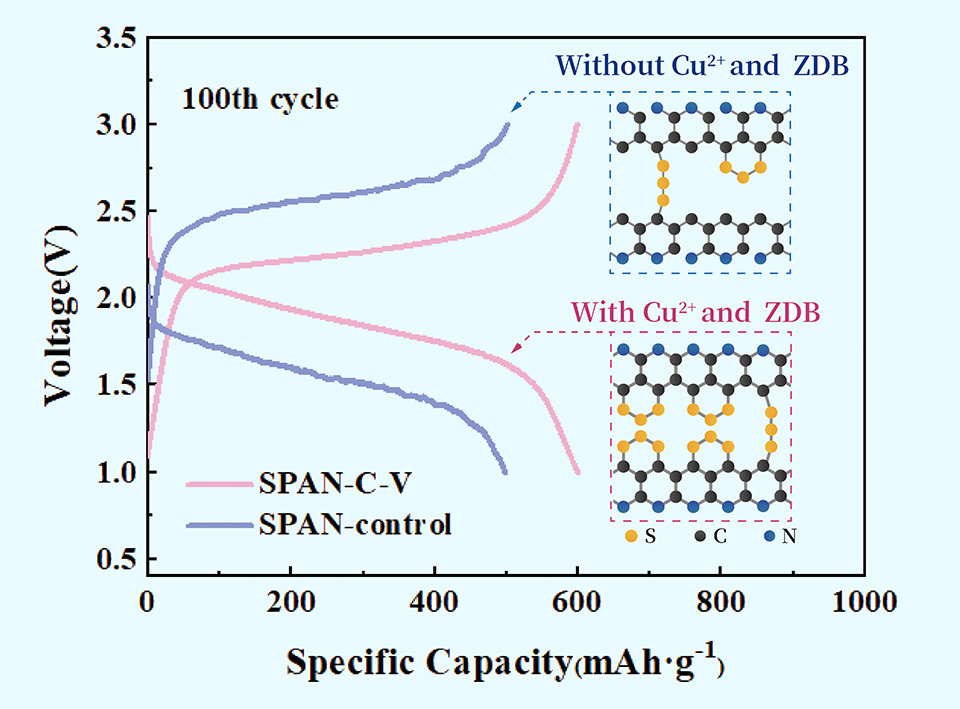
硫化聚丙烯腈因其不溶解机制和有效缓解锂硫电池中多硫化物“穿梭效应”,被认为是具有吸引力的锂硫电池正极候选材料。硫化聚丙烯腈的导电聚合物骨架具有优异的电子导电性,同时共轭主链能有效解决充放电过程中硫正极体积变化引起的正极结构坍塌问题。因硫化聚丙烯腈的固-固反应机理,有效克服了传统硫正极在醚类电解液中多硫化物溶解及穿梭效应的问题,具有高正极活性物质利用率、出色的循环稳定性和结构稳定性等优势。有许多研究工作致力于通过硫化促进剂来提高硫化聚丙烯腈的硫含量,进而提高材料的能量密度。其中,硫化聚丙烯腈主链的环化度与循环稳定性的关系引起了我们的关注。在该研究工作中,通过在硫化过程中引入无水硫酸铜和正乙基正苯基二硫代氨基甲酸锌(ZDB)合成了SPAN-C-V复合材料。无水硫酸铜和ZDB的共同引入降低了聚丙烯腈环化反应的起始温度,同时提高了产物SPAN-C-V内碳碳双键的含量,在提高了材料硫含量的同时提高了其环化度。以SPAN-C-V为正极活性物质所组装的锂硫电池展现出良好的循环稳定性和倍率性能:在0.2 C (1 C = 600 mAh·kg-1)下循环100次后的可逆容量为601 mAh·kg-1,容量保持率为93%。该工作对于硫化聚丙烯腈材料的发展提供了参考。
关键词: 硫化聚丙烯腈; CuSO4; 正乙基正苯基二硫代氨基甲酸锌; 环化度; 锂硫电池
姬璇 , 汪佳裕 , 王安邦 , 王维坤 , 姚明 , 黄雅钦 . 锂硫电池用高度环化硫化聚丙烯腈的制备[J]. 电化学, 2022 , 28(12) : 2219010 . DOI: 10.13208/j.electrochem.2219010
Sulfurized polyacrylonitrile (SPAN) is regarded as an attractive cathode candidate of lithium-sulfur (Li-S) batteries for its non-dissolution mechanism and effective alleviation of polysulfides shuttling issue in Li-S batteries, displaying high utilization of cathode active material, outstanding cycle stability and structural stability. However, the relation between cyclization degree and cycle stability of SPAN is still unveiled. In this work, SPAN-C-V composites were synthesized by co-introduction of CuSO4 and zinc n-ethyl-n-phenyldithiocarbamate (ZDB) in the co-heating of sulfur and polyacrylonitrile. The co-introduction of CuSO4 and ZDB reduced the cyclization reaction onset temperature of PAN while increased the C—C/C=C within SPAN-C-V, thus led to an increase in the degree of cyclization of SPAN-C-V, achieving excellent electrochemical performance by simultaneously improving the cyclization degree and increasing the content of sulfur. The SPAN-C-V exhibited an initial reversible capacity of 805 mAh·g-1 and 601 mAh·g-1 after 100 cycles with the capacity retention rate of 93% at 0.2 C (1 C = 600 mAh·g-1). The focus on the cyclization degree of SPAN provides an enlightenment of advanced cathode material.

/
| 〈 |
|
〉 |