

多电子反应材料推动高能量密度电池发展:材料与体系创新
收稿日期: 2022-09-21
修回日期: 2022-10-25
网络出版日期: 2022-11-14
Multi-Electron Reaction-Boosted High Energy Density Batteries: Material and System Innovation
Received date: 2022-09-21
Revised date: 2022-10-25
Online published: 2022-11-14
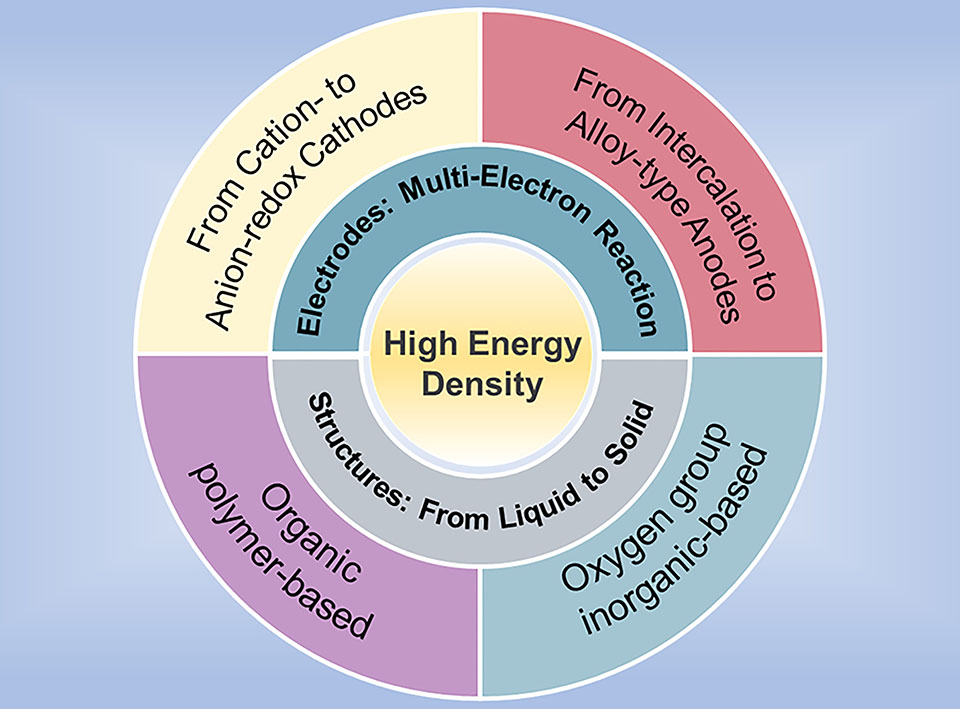
全球能源结构转型推动了电化学储能系统的飞速发展,提高能量密度是发展新型二次电池的重要方向和研究热点。然而,受限于传统的嵌入式反应,锂离子电池在能量密度上已经逐渐达到极限。要发展更高能量密度的新型二次电池,需要在新理论、新材料和新体系上进行突破。基于此,本文总结了20年来多电子反应材料概念的形成、理论的发展、材料创制的历程。在“轻元素多电子反应”和“多离子效应”核心设计准则的指导下,具有上述特征的电极材料与电池结构不断发展迭代,引领了高能量密度电池的发展方向。从阳离子氧化还原到阴阳离子协同氧化还原,从嵌入式反应到合金化反应,从传统有机液态体系到电池固态化,本文梳理了典型的多电子反应正负极材料的结构特性、体系创新和工程化前景,剖析了多电子反应电极材料的瓶颈问题,并分析了电池固态化发展所面临的挑战。最后,对高能量密度电池的未来发展趋势和难点进行了归纳与展望。
郭瑞琪 , 吴锋 , 王欣然 , 白莹 , 吴川 . 多电子反应材料推动高能量密度电池发展:材料与体系创新[J]. 电化学, 2022 , 28(12) : 2219011 . DOI: 10.13208/j.electrochem.2219011
The continuous development of the global energy structure transformation has put forward higher demands upon the development of batteries. The improvements of the energy density have become one of the important indicators and hot topic for novel secondary batteries. The energy density of existing lithium-ion battery has encountered a bottleneck due to the limitations of material and systems. Herein, this paper introduces the concept and development of multi-electron reaction materials over the past twenty years. Guided by the multi-electron reaction, light weight electrode and multi-ion effect, current development strategies and future trends of high-energy-density batteries are highlighted from the perspective of materials and structure system innovation. Typical cathode and anode materials with the multi-electron reactions are summarized from cation-redox to anion-redox, from intercalation-type to alloying-type, and from liquid systems to solid-state lithium batteries. The properties of the typical materials and their engineering prospects are comprehensively discussed, and additionally, the application potential and the main challenges currently encountered by solid-state batteries are also introduced. Finally, this paper gives a comprehensive outlook on the development of high-energy-density batteries.

/
| 〈 |
|
〉 |