

紫外光引发原位交联多功能粘结剂构筑稳固硫正极
收稿日期: 2022-07-30
修回日期: 2022-09-13
录用日期: 2022-11-04
网络出版日期: 2022-11-07
Ultraviolet-Initiated In-Situ Cross-Linking of Multifunctional Binder Backbones Enables Robust Lithium-Sulfur Batteries
Received date: 2022-07-30
Revised date: 2022-09-13
Accepted date: 2022-11-04
Online published: 2022-11-07
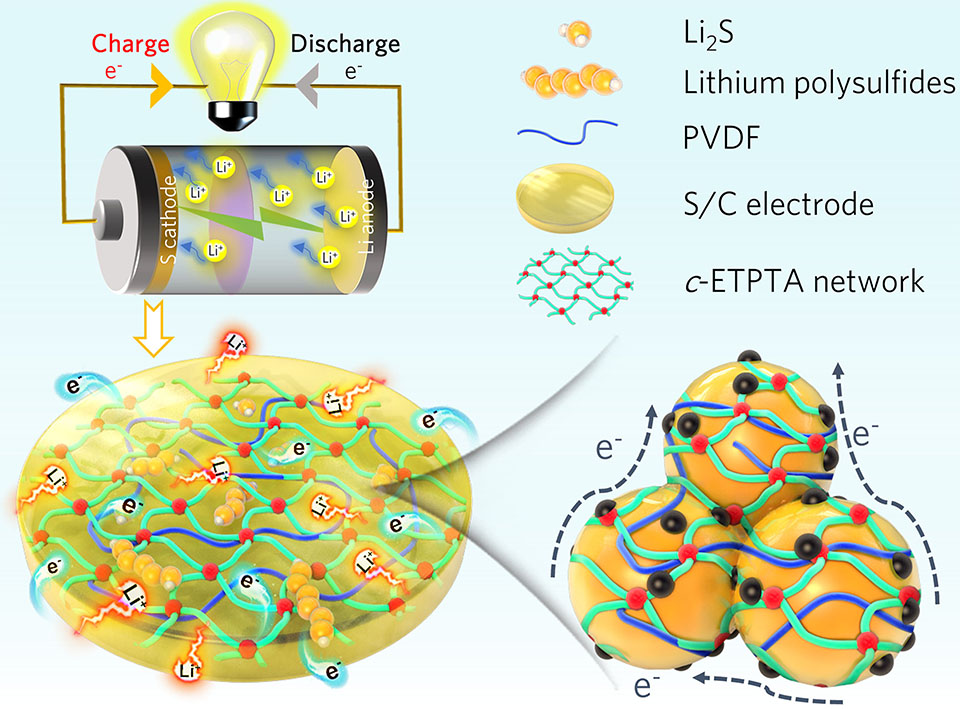
锂硫电池因其高理论比容量和高能量密度的独特优势,在下一代储能体系中展现出重要的应用前景。然而,锂硫电池的商业化进程仍面临诸多挑战:如可溶性多硫化锂中间产物造成的“穿梭”问题、充放电过程中体积变化剧烈以及电极硫负载增大时的严重极化等,易导致硫正极的结构坍塌和电化学性能的快速衰变。电池作为一个有机整体,其性能优化是一个系统工程,上述挑战对电池内的每一个组分都提出了更高的要求,例如发展具有更好机械性能的新型粘结剂。在本工作中,我们首次在硫正极中引入乙氧基化三羟甲基丙烷三丙酸酯单体,通过紫外光辅助固化实现原位交联,并与传统聚偏氟乙烯粘结剂构成二元粘结剂(简称c-ETPTA/PVDF),用于制备高强度、高硫负载的长寿命锂硫电池。结果表明,采用共价交联的c-ETPTA/PVDF粘结剂不但能显著增强电极极片的机械性能,保持循环过程中的结构稳定性,还可借助其丰富的含氧官能团对溶解性多硫化锂中间产物进行高效地捕获。此外,c-ETPTA/PVDF中的醚氧键与锂离子之间适度的相互作用也有助于锂离子的快速输送。因此,S-c-ETPTA/PVDF电极在2 C倍率下可稳定循环1000次以上,且每个周期的容量衰减率仅为0.038%。即使当硫面载量提高至7.8 mgS·cm-2时,经过50个周期循环后,仍可输出6.2 mAh·cm-2的高平均放电面容量。本工作展示了紫外光引发原位交联技术在制备稳固的高能量密度锂硫电池方面的巨大应用前景。
李莎 , 湛孝 , 王顾莲 , 王慧群 , 熊伟明 , 张力 . 紫外光引发原位交联多功能粘结剂构筑稳固硫正极[J]. 电化学, 2023 , 29(4) : 2217004 . DOI: 10.13208/j.electrochem.2217004
Lithium-sulfur (Li-S) batteries show attractive prospects owing to their high theoretical energy density, but their commercialization still faces such challenges as lithium polysulfides shuttling, severe volume change and considerable polarization. These stubborn issues place higher demands on each component in the battery, such as the development of multifunctional binders with superior mechanical properties. Herein, ethoxylated trimethylolpropane triacrylate was firstly introduced into sulfur cathodes, and in-situ cross-linked by ultraviolet (UV) curing combined with traditional polyvinylidene difluoride binder (i.e., forming a binary binder, denoted as c-ETPTA/PVDF) to construct high-loading and durable Li-S batteries. The covalently cross-linked ETPTA framework not only significantly enhances the mechanical strength of the laminate, but also offers a strong chemical affinity for lithium polysulfides due to the abundant oxygen-containing groups. Moreover, the moderate interaction force between ether oxygen bonds and Li+ further accelerates the Li+ transport. As such, the S-c-ETPTA/PVDF electrode exhibited an ultralow attenuation rate of 0.038% at 2 C over 1000 cycles. Even under a sulfur loading of 7.8 mgS·cm-2, an average areal capacity of 6.2 mAh·cm-2 could be achieved after 50 cycles. This work indicates that light-assisted curing technology holds great promise in the fabrication of robust and high-energy-density Li-S batteries.

/
| 〈 |
|
〉 |