

无序Ru-O构型对电化学析氢催化性能研究
收稿日期: 2022-07-09
修回日期: 2022-08-28
网络出版日期: 2022-09-30
Catalytic Effect of Disordered Ru-O Configurations for Electrochemical Hydrogen Evolution
Received date: 2022-07-09
Revised date: 2022-08-28
Online published: 2022-09-30
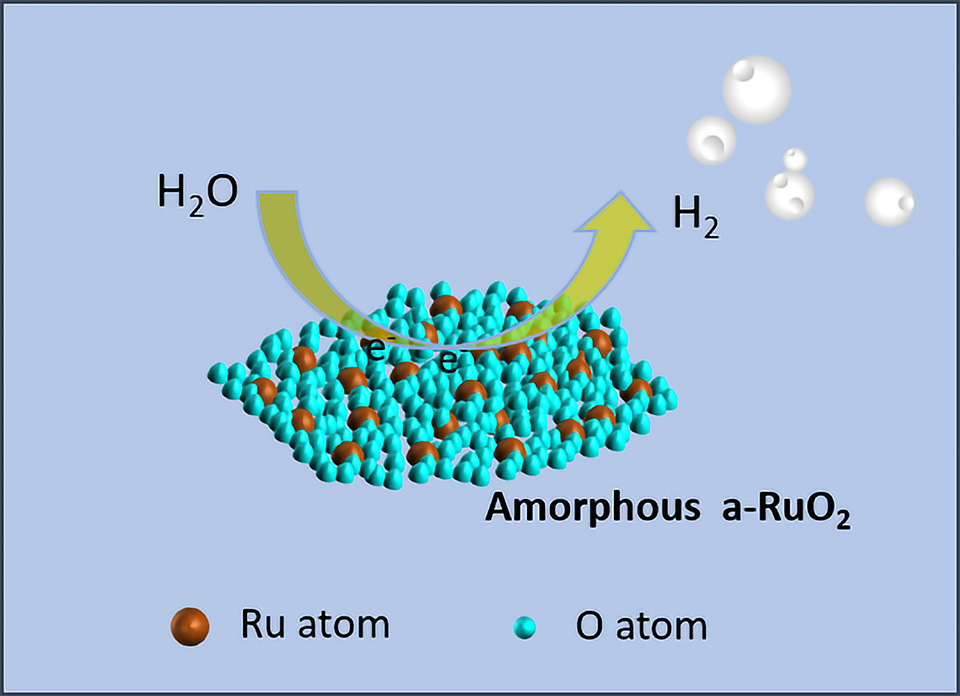
相工程被认为是调节催化剂电子结构和催化活性的有效方法。非晶材料的无序构型允许表面电子结构的灵活重整,显示出其作为析氢反应(HER)催化剂的吸引力。在此,我们设计并开发了一种具有无序Ru-O构型的非晶催化剂(a-RuO2)。结合先进的电镜技术和详细的电化学测试,建立了Ru-O有序性与HER性能的构效关系。具体来说,无序的Ru-O配位显著增强了酸性和碱性 HER 中的催化活性,最终使经济性更高的a-RuO2催化性能接近商业Pt/C。此外,在10 mA·cm-2下进行10 h电流-时间(i-t)测试后,a-RuO2表现出极好的稳定性。进一步的理论模拟显示a-RuO2较低的d带中心和优化的电子输运调制了活性位点对中间反应物的吸附强度,促进了HER动力学。这项工作为通过相工程探索高活性HER催化剂提供了新的观点。
孙雪 , 宋亚杰 , 李仁龙 , 王家钧 . 无序Ru-O构型对电化学析氢催化性能研究[J]. 电化学, 2022 , 28(10) : 2214011 . DOI: 10.13208/j.electrochem.2214011
Phase engineering is considered as an effective method for modulating the electronic structure and catalytic activity of catalysts. The disordered conformation of amorphous materials allows flexible reforming of the surface electronic structure, showing their attractiveness as catalysts for hydrogen evolution reaction (HER). Herein, we designed and developed an amorphous ruthenium dioxide (a-RuO2) catalyst with a disordered Ru-O configuration. The conformational relationship between Ru-O ordering and HER performance is established by combining advanced electron microscopic techniques with detailed electrochemical tests. Specifically, the disordered Ru-O coordination significantly enhanced the HER catalytic activity in both acidic and alkaline media, ultimately leading to HER performance of a-RuO2 approaching that of commercial Pt/C with higher economics. In addition, a-RuO2 exhibited excellent stability after 10 h current-time (i-t) testing at 10 mA·cm-2. Further theoretical simulations showed that the lowered d-band center and optimized electron transport of a-RuO2 modulated the adsorption strength of the active site to the intermediate reactants, promoting HER kinetics. This work provides a new perspective for exploring highly active HER catalysts through phase engineering.

/
| 〈 |
|
〉 |