

基于阳极甘油氧化电催化的碱/酸混合电解制氢研究
收稿日期: 2022-06-01
修回日期: 2022-06-23
录用日期: 2022-08-29
网络出版日期: 2022-08-31
Anodic Electrocatalysis of Glycerol Oxidation for Hybrid Alkali/Acid Electrolytic Hydrogen Generation
Received date: 2022-06-01
Revised date: 2022-06-23
Accepted date: 2022-08-29
Online published: 2022-08-31
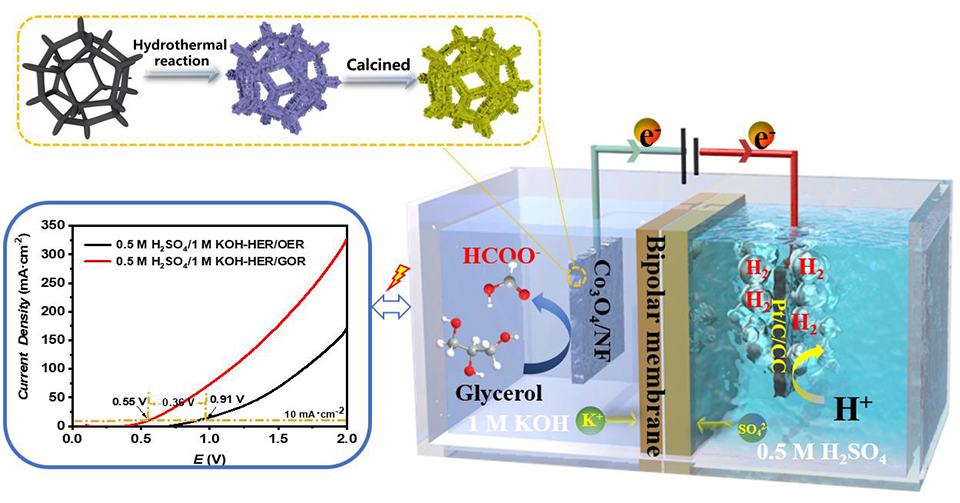
耦合可再生电能的电解水制氢是一项极具前景的绿氢技术,该技术仍受限于阳极析氧反应(OER)动力学慢、过电位高等问题的限制。在阳极端采用热力学更容易的电氧化反应代替OER,可大幅降低电耗并且在阳极端获得增值产物,是电解制氢的一种新策略。甘油作为生物柴油生产的主要副产品且产能过剩,其电催化氧化(GOR)理论电位比OER低。基于此,本研究工作报道了一种耦合酸性析氢反应(HER)与碱性GOR的混合酸/碱双电解液的制氢电解器,其以泡沫镍(NF)支撑Co3O4纳米片(NS)电极(Co3O4·NSs/NF)为阳极,商用碳载铂修饰碳布电极为阴极。在阳极端,Co3O4·NSs/NF对GOR电催化表现出较低的过电位和转化为甲酸盐的高选择性。在该混合酸/碱双电解液电解槽中,仅仅需要额外施加0.55 V的外加电压,即可达到10 mA·cm-2的产氢电解电流密度,并可以在阳极将甘油高选择性地转化为甲酸盐,其中产氢的法拉第效率接近100%。本研究工作为电解制氢提供了一条节电、阳极增值转化的技术路线。
冯辛 , 刘博文 , 郭可鑫 , 范林丰 , 王根香 , 次素琴 , 温珍海 . 基于阳极甘油氧化电催化的碱/酸混合电解制氢研究[J]. 电化学, 2023 , 29(2) : 2215005 . DOI: 10.13208/j.electrochem.2215005
Electrolytic hydrogen production is heavily restricted by high-energy consumption majorly due to the relatively high potential of anodic oxygen evolution reaction (OER). Development of OER-alternative reaction at the anode has been recently proposed as a promising pathway to address the associated issues. In this work, we report a hybrid acid/alkali dual-electrolyte electrolyzer by coupling acidic hydrogen evolution reaction (HER) using commercial Pt/C cathode with alkaline electrocatalytic glycerol oxidation (GOR) which is implemented by developing a nickel foam (NF) supporting Co3O4 nanosheets anode that shows low overpotential and high selectivity toward GOR for formate production. The hybrid acid/alkali electrolyzer only requires an applied voltage of 0.55 V to achieve the electrolytic current density of 10 mA·cm-2 for glycerol conversion into formate at the anode and H2 production at the cathode with the Faraday efficiency of about 100%. The present work may open a new avenue to maximize the electron utilization efficiency and implement the energy-saving green route for H2 generation.

/
| 〈 |
|
〉 |