

焦耳热快速合成双功能电催化剂用于高效水分解
The Rapid Preparation of Efficient MoFeCo-Based Bifunctional Electrocatalysts via Joule Heating for Overall Water Splitting
Received date: 2022-06-28
Revised date: 2022-07-21
Online published: 2022-08-23
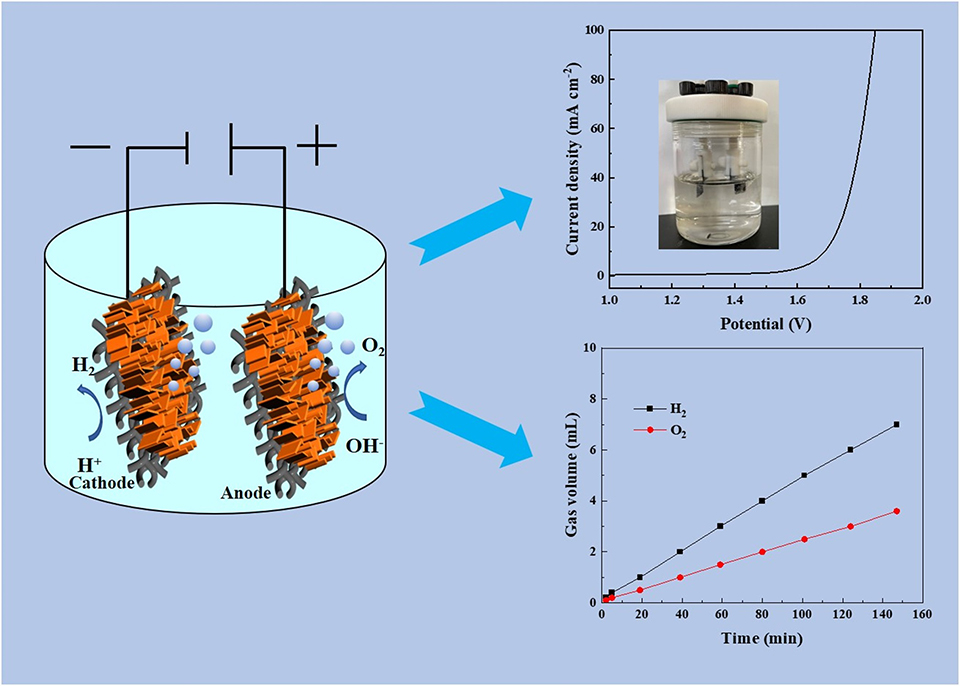
电解水是有效的产氢方式之一, 开发具有高催化活性的电极材料是当前电解水的研究热点,但仍面临诸多挑战。 本研究报告了一种通过焦耳热技术快速制备多金属异质结构, 并将其用作电解水的双功能电催化剂, 展现出优异的电解水催化活性。通过焦耳热处理三种金属前驱涂覆的碳布, Mo2C和CoO/Fe3O4异质结构形成。当其用作析氢(HER)和析氧(OER)的双功能催化剂时, 仅需121 mV和268 mV的过电位,可以实现10 mA·cm-2的电流密度。当用于两电极电解水时, MoC/FeO/CoO/CC作为阳极和阴极催化剂表现出优异的电催化性能和长期稳定性, 仅需1.69 V即可实现10 mA·cm-2的电流密度, 并且展现出25小时的稳定性。本研究通过简单、 快速的焦耳热技术实现了双金属/多金属异质结构的构筑,并应用于高效水电解,为合理设计多金属异质结构提供指导。
周澳 , 郭伟健 , 王月青 , 张进涛 . 焦耳热快速合成双功能电催化剂用于高效水分解[J]. 电化学, 2022 , 28(9) : 2214007 . DOI: 10.13208/j.electrochem.2214007
Water electrolysis is an available way to obtain green hydrogen. The development of highly efficient electrocatalysts is a current research hotspot for water splitting, but it remains challenging. Herein, we demonstrate the synthesis of a robust bifunctional multi-metal electrocatalysts toward water splitting via the rapid Joule-heating conversion of metal precursors. The composition and morphology were well regulated via altering the ratio of metal precursors. In particular, the trimetal MoC/FeO/CoO/carbon cloth (CC) electrode revealed the outstanding bifunctional electrocatalytic performance due to the unique composition and large electrochemical active surface area. Typically, the MoC/FeO/CoO/CC catalyst needed low overpotentials of 121 and 268 mV to reach 10 mA·cm-2 toward HER and OER in 1 mol·L-1 KOH solution, respectively. When used as both cathode and anode, a small potential of 1.69 V was required to achieve 10 mA·cm-2 for overall water splitting and an impressive stability for 25 h was observed. This facile and rapid Joule heating strategy offers guideline for rational manufacture of bimetal or multi-metal electrocatalysts toward diverse application.

| [1] | Cai S H, Chen X N, Huang M J, Han J Y, Zhou Y W, Li J S. Interfacial engineering of nickel/iron/ruthenium phosphides for efficient overall water splitting powered by solar energy[J]. J. Mater. Chem. A., 2022, 10(2): 772-778. |
| [2] | Chang J F, Wang G Z, Yang Z Z, Li B Y, Wang Q, Kuliiev R, Orlovskaya N, Gu M, Du Y G, Wang G F, Yang Y. Dual-doping and synergism toward high-performance seawater electrolysis[J]. Adv. Mater., 2021, 33(33): 2101425. |
| [3] | Wang Y Q, Ma J Z, Wang J, Chen S, Wang H S, Zhang J T. Interfacial scaffolding preparation of hierarchical PBA-based derivative electrocatalysts for efficient water splitting[J]. Adv. Energy Mater., 2019, 9(5): 1802939. |
| [4] | Wang Y Q, Zhang B H, Pan W, Ma H Y, Zhang J T. 3 D porous nickel-cobalt nitrides supported on nickel foam as efficient electrocatalysts for overall water splitting[J]. Chem-SusChem, 2017, 10(21): 4170-4177. |
| [5] | Gautam J, Liu Y, Gu J, Ma Z Y, Zha J J, Dahal B, Zhang L N, Chishti A N, Ni L B, Diao G W, Wei Y G. Fabrication of polyoxometalate anchored zinc cobalt sulfide nanowires as a remarkable bifunctional electrocatalyst for overall water splitting[J]. Adv. Funct. Mater., 2021, 31(46): 2106147. |
| [6] | Gu M Z, Deng X Y, Lin M, Wang H, Gao A, Huang X M, Zhang X J. Ultrathin NiCo bimetallic molybdate nano-sheets coated CuOx nanotubes: Heterostructure and bimet-allic synergistic optimization of the active site for highly efficient overall water splitting[J]. Adv. Energy Mater., 2021, 11(41): 2102361. |
| [7] | Kim Y, Kim D, Lee J, Lee L. Tuning the electrochemical properties of polymeric cobalt phthalocyanines for efficient water splitting[J]. Adv. Funct. Mater., 2021, 31(41): 2103290. |
| [8] | Yang H, Liu Y F, Liu X L, Wang X K, Tian H, Waterhouse G, Kruger P, Telfer S, Ma S Q. Large-scale synthesis of N-doped carbon capsules supporting atomically dispersed iron for efficient oxygen reduction reaction electrocatalysis[J]. eScience, 2022, 2: 227-234. |
| [9] | Li S S, Wang L, Su H, Hong A N, Wang Y X, Yang H J, Ge L, Song W Y, Liu J, Ma T Y, Bu X H, Feng P Y. Electron redistributed S-doped nickel iron phosphides derived from one-step phosphatization of MOFs for significantly boosting electrochemical water splitting[J]. Adv. Funct. Mater., 2022, 32(23): 2200733. |
| [10] | Li W, Liu J, Guo P F, Li H Z, Fei B, Guo Y H, Pan H G, Sun D L, Fang F, Wu R B. Co/CoP heterojunction on hierarchically ordered porous carbon as a highly efficient electrocatalyst for hydrogen and oxygen evolution[J]. Adv. Energy Mater., 2021, 11(42): 2102134. |
| [11] | Lin Z P, Xiao B B, Wang Z P, Tao W Y, Shen S J, Huang L A, Zhang J T, Meng F Q, Zhang Q H, Gu L, Zhong W W. Planar-coordination PdSe2 nanosheets as highly active electrocatalyst for hydrogen evolution reaction[J]. Adv. Funct. Mater., 2021, 31(32): 2102321. |
| [12] | Liu C, Qian J, Ye Y F, Zhou H, Sun C J, Sheehan C, Zhang Z Y, Wan G, Liu Y S, Guo J H, Li S, Shin H, Hwang S, Gunnoe T B, Goddard W A, Zhang S. Oxygen evolution reaction over catalytic single-site Co in a well-defined brookite TiO2 nanorod surface[J]. Nat. Catal., 2021, 4(1): 36-45. |
| [13] | Liu H X, Li X Y, Chen L L, Zhu X D, Dong P, Chee M, Ye M X, Guo Y H, Shen J F. Monolithic Ni-Mo-B bifunctional electrode for large current water splitting[J]. Adv. Funct. Mater., 2022, 32(4): 2107308. |
| [14] | Li Y, Jiang K Y, Yang J, Zheng Y Y, Hubner R, Ou Z W, Dong X, He L Q, Wang H L, Li J, Sun Y J, Lu X B, Zhuang X D, Zheng Z K, Liu W. Tungsten oxide/reduced graphene oxide aerogel with low-content platinum as high-performance electrocatalyst for hydrogen evolution reaction[J]. Small, 2021, 17(37): 2102159. |
| [15] | Peng Y, Liu Q M, Lu B Z, He T, Nichols F, Hu X, Huang T, Huang G, Guzman L, Ping Y, Chen S W. Organically capped iridium nanoparticles as high-performance bifunctional electrocatalysts for full water splitting in both acidic and alkaline media: Impacts of metal-ligand interfacial interactions[J]. ACS Catal., 2021, 11(3): 1179-1188. |
| [16] | Shan J Q, Ye C, Chen S M, Sun T L, Jiao Y, Liu L M, Zhu C Z, Song L, Han Y, Jaroniec M, Zhu Y H, Zheng Y, Qiao S Z. Short-range ordered iridium single atoms integrated into cobalt oxide spinel structure for highly efficient electrocatalytic water oxidation[J]. J. Am. Chem. Soc., 2021, 143(13): 5201-5211. |
| [17] | Wang H M, Chen Z N, Wu D S, Cao M N, Sun F F, Zhang H, You H H, Zhuang W, Cao R. Significantly enhanced overall water splitting performance by partial oxidation of Ir through Au modification in core-shell alloy structure[J]. J. Am. Chem. Soc., 2021, 143(12): 4639-4645. |
| [18] | Wang M J, Xu Y, Peng C K, Chen S Y, Lin Y G, Hu Z W, Sun L, Ding S Y, Pao C W, Shao Q, Huang X Q. Site-specified two-dimensional heterojunction of Pt nanoparticles/metal-organic frameworks for enhanced hydrogen evolution[J]. J. Am. Chem. Soc., 2021, 143(40): 16512-16518. |
| [19] | Wang T X, Guo L L, Jiang Z, Chen S T, Xu S R, Zhang Y X, Zhang J, Li R J, Peng T Y. Ru-Pincer complex-bridged Cu-porphyrin polymer for robust (photo)electrocatalytic H2 evolution via single-atom active sites[J]. Adv. Funct. Mater., 2021, 31(50): 2107290. |
| [20] | Wang X D, Wang C, Lai F Y, Sun H X, Yu N, Geng B Y. Self-supported CoFe-P nanosheets as a bifunctional catalyst for overall water splitting[J]. ACS Appl. Nano Mater., 2021, 4(11): 12083-12090. |
| [21] | Zhang Q, Xiao W, Guo W H, Yang Y X, Lei J L, Luo H Q, Li N B. Macroporous array induced multiscale modulation at the surface/interface of Co(OH)2/NiMo self-supporting electrode for effective overall water splitting[J]. Adv. Funct. Mater., 2021, 31(33): 2102117. |
| [22] | Liu J C, Wang Y, Liao Y F, Wu C L, Yan Y G, Xie H J, Chen Y G. Heterostructured Ni3S2-Ni3P/NF as a bifunctional catalyst for overall urea-water electrolysis for hydrogen generation[J]. ACS Appl. Mater. Interfaces., 2021, 13(23): 26948-26959. |
| [23] | Riyajuddin S, Azmi K, Pahuja M, Kumar S, Maruyama T, Bera C, Ghosh K. Super-Hydrophilic hierarchical Ni-foam-graphene-carbon nanotubes-Ni2P-CuP2 nano-architecture as efficient electrocatalyst for overall water splitting[J]. ACS Nano., 2021, 15(3): 5586-5599. |
| [24] | Tang Y J, Zou Y, Zhu D D. Efficient water oxidation using an Fe-doped nickel telluride-nickel phosphide electrocatalyst by partial phosphating[J]. J. Mater. Chem. A., 2022. |
| [25] | Wang X Y, Xie Y, Zhou W, Wang X W, Cai Z C, Xing Z P, Li M X, Pan K. The self-supported Zn-doped CoNiP microsphere/thorn hierarchical structures as efficient bifunctional catalysts for water splitting[J]. Electrochim. Acta., 2020, 339: 135933. |
| [26] | Zhang B, Shan J W, Wang W L, Tsiakaras P, Li Y Y. Oxygen vacancy and core-shell heterojunction engineering of anemone-like CoP@CoOOH bifunctional electrocatalyst for efficient overall water splitting[J]. Small, 2022, 18(12): 2106012. |
| [27] | Zhang B, Zheng Y J, Ma T, Yang C D, Peng Y F, Zhou Z H, Zhou M, Li S, Wang Y H, Cheng C. Designing MOF nanoarchitectures for electrochemical water splitting[J]. Adv. Mater., 2021, 33(17): 2006042. |
| [28] | Lv Z, Ma W S, Wang M, Dang J, Jian K L, Liu D, Huang D J. Co-constructing interfaces of multiheterostructure on Mxene (Ti3C2Tx)-modified 3d self-supporting electrode for ultraefficient electrocatalytic HER in alkaline media[J]. Adv. Funct. Mater., 2021, 31(29): 2102576. |
| [29] | Chen Y, Liu Q, Yao Y, Wu H B, Wang Y X, Zheng H Q, Cui Y J, Qian G D. Engineering different reaction centers on hierarchical Ni/NiFe layered double hydroxide acc-elerating overall water splitting[J]. ACS Appl. Energy Mater., 2021, 4(9): 9858-9865. |
| [30] | Dai Z F, Geng H B, Wang J, Luo Y B, Li B, Zong Y, Yang J, Guo Y Y, Zheng Y, Wang X, Yan Q Y. Hexagonal-phase cobalt monophosphosulfide for highly efficient overall water splitting[J]. ACS Nano, 2017, 11(11): 11031-11040. |
| [31] | Wang X, Zhang L, Liu C P, Ge J J, Zhu J B, Xing W. Recent advances in structural regulation on non-precious metal catalysts for oxygen reduction reaction in alkaline electrolytes[J]. J. Electrochem., 2022, 28(2): 2108501. |
| [32] | Jiang X, Xie Q F, Lu G X, Wang Y, Liu T F, Liu Y J, Tao X Y, Nai J W. Synthesis of NiSe2/Fe3O4 nanotubes with heteroepitaxy configuration as a high-efficient oxygen evolution electrocatalyst[J]. Small Methods, 2022: 2200377. |
| [33] | Li L, Guo Y, Wang X R, Liu X W, Lu Y W. Ultraeven Mo-doped CoP nanocrystals as bifunctional electrocatalyst for efficient overall water splitting[J]. Langmuir, 2021, 37(19): 5986-5992. |
| [34] | Chen Q W, Chen S, Zhao L L, Ma J Z, Wang H S, Zhang J T. Interface coating of iron nitride on carbon cloth for reversible lithium redox in rechargeable battery[J]. Chem. Eng. J., 2022, 431 (1), 133961. |
| [35] | Cao X Y, Tan D X, Wulan B R, Zhang J T. In situ characterization for boosting electrocatalytic carbon dioxide reduction[J]. Small Methods, 2021, 5(10): 2100700. |
| [36] | Chen Y N, Egan G C, Wan J Y, Zhu S Z, Jacob R J, Zhou W B, Dai J Q, Wang Y B, Danner V A, Yao Y G, Fu K, Wang Y B, Bao W Z, Li T, Zachariah M R, Hu L B. Ultra-fast self-assembly and stabilization of reactive nanoparticles in reduced graphene oxide films[J]. Nat. Commun., 2016, 7(1): 1-9. |
| [37] | Yao Y G, Huang Z N, Xie P F, Lacey S D, Jacob R J, Xie H, Chen F J, Nie A, Pu T C, Rehwoldt M, Yu D W, Zachariah M, Wang C, Li J, Hu L B. Carbothermal shock synthesis of high-entropy-alloy nanoparticles[J]. Science, 2018, 359(6383): 1489-1494. |
| [38] | Wu H, Lu Q, Zhang J F, Wang J J, Han X P, Zhao N Q, Hu W B, Li J J, Chen Y N, Deng Y D. Thermal shock-activated spontaneous growing of nanosheets for overall water splitting[J]. Nanomicro Lett., 2020, 12(1): 1-12. |
| [39] | Chen P Z, Xu K, Fang Z W, Tong Y, Wu J C, Lu X L, Peng X, Ding H, Wu C Z, Xie Y. Metallic CO4N porous nanowire arrays activated by surface oxidation as electrocatalysts for the oxygen evolution reaction[J]. Angew. Chem. Int. Ed., 2015, 54(49): 14710-14714. |
| [40] | Dai Z F, Geng H B, Wang J, Luo Y B, Li B, Zong Y, Yang J, Guo Y Y, Zheng Y, Wang X, Yan Q Y. Hexagonal-phase cobalt monophosphosulfide for highly efficient overall water splitting[J]. ACS Nano, 2017, 11(11): 11031-11040. |
| [41] | Wang P C, Liu X F, Yan Y T, Cao J, Feng J C, Qi J L. Exploring CoP core-shell nanosheets by Fe and Zn dual cation doping as efficient electrocatalysts for overall water splitting[J]. Catal. Sci. Technol., 2020, 10(5): 1395-1400. |
| [42] | Liu T, Li P, Yao N, Cheng G Z, Chen S L, Luo W, Yin Y D. CoP-doped MOF-based electrocatalyst for pH-universal hydrogen evolution reaction[J]. Angew. Chem. Int. Ed., 58(14): 4679-4684. |
| [43] | Lou H, Yu T, Ma J N, Zhang S T, Bergara A, Yang G C. Achieving high hydrogen evolution reaction activity of a Mo2C monolayer[J]. Phys. Chem. Chem. Phys., 2020, 22: 26189-26199. |
| [44] | Zhang L Y, Zheng Y J, Wang J C, Geng Y, Zhang B, He J J, Xue J M, Frauenheim T, Li M. Ni/Mo bimetallic-oxide-derived heterointerface-rich sulfide nanosheets with Co-doping for efficient alkaline hydrogen evolution by boosting volmer reaction[J]. Small, 2021, 17(10): 2006730. |
| [45] | Wan J, Wu J B, Gao X, Li T Q, Hu Z M, Yu H M, Huang L. Structure confined porous Mo2C for efficient hydrogen evolution[J]. Adv. Funct. Mater., 2017, 27(45): 1703933. |
| [46] | Ma H B, Chen Z W, Wang Z L, Singh C V, Jiang Q. Interface engineering of Co/CoMoN/NF heterostructures for high-performance electrochemical overall water splitting[J]. Adv. Sci., 2022, 9(11): 2105313. |
| [47] | Wang L, Fan J Y, Liu Y, Chen M Y, Lin Y, Bi H C, Liu B X, Shi N E, Xu D D, Bao J C, Han M. Phase-modulation of iron/nickel phosphides nanocrystals “armored”with porous P-doped carbon and anchored on P-doped graphene nanohybrids for enhanced overall water splitting[J]. Adv. Funct. Mater., 2021, 31(30): 2010912. |
| [48] | Wang L, Fan J Y, Liu Y, Chen M Y, Lin Y, Bi H C, Liu B X, Shi N E, Xu D D, Bao J C, Han M. Phase-modulation of iron/nickel phosphides nanocrystals “armored”with porous P-doped carbon and anchored on P-doped graphene nanohybrids for enhanced overall water splitting[J]. Adv. Funct. Mater., 2021, 31(30): 2010912. |
| [49] | Zhou X F, Tian Y H, Luo J, Jin B, Wu Z J, Ning X M, Zhan L, Fan X L, Zhou T, Zhang S Q, Zhou X S. MoC quantum dots@N-doped-carbon for low-cost and efficient hydrogen evolution reaction: From electrocatalysis to photocatalysis[J]. Adv. Funct. Mater., 2022: 2201518. |
| [50] | Cai F F, Guo Y J, Ibrahim J J, Zhang J, Sun Y H. A highly active and stable Pd/MoC catalyst for hydrogen production from methanol decomposition[J]. Appl. Catal. B-Environ., 2021, 299: 120648. |
/
| 〈 |
|
〉 |