

碱性电解水高效制氢
Alkaline Water Electrolysis for Efficient Hydrogen Production
Received date: 2022-06-29
Revised date: 2022-07-12
Online published: 2022-08-03
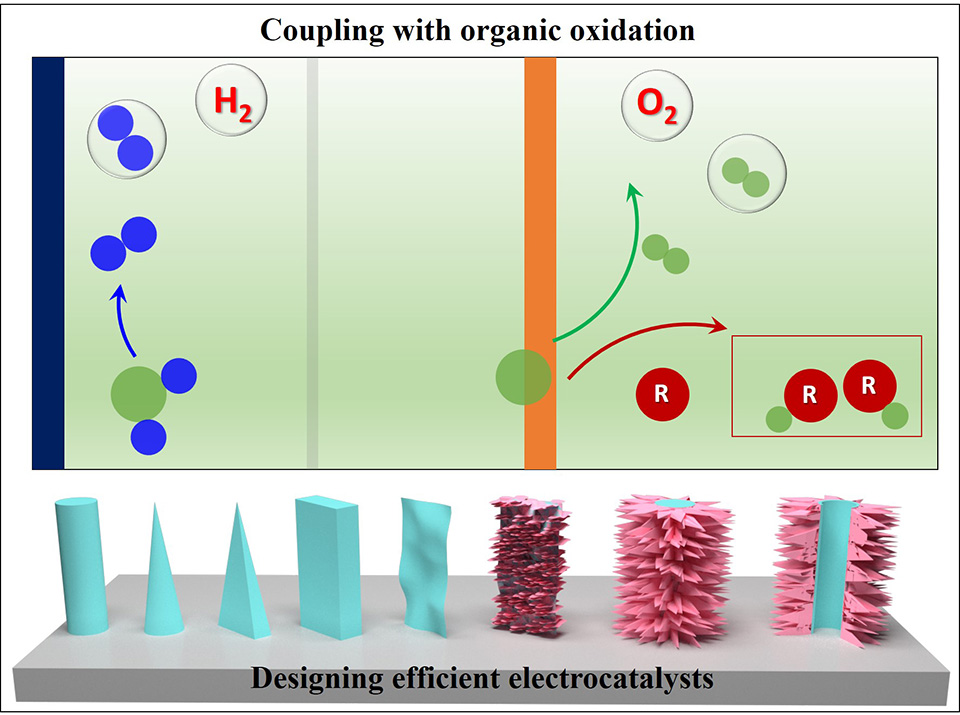
与传统化石能源制氢技术相比,利用可再生能源驱动电解水制氢技术具有绿色可持续和制氢效率高等优势,被认为是目前最具前景的制氢方式。然而, 由于电解水两极反应动力学缓慢、 催化剂稳定性较差, 限制了其大规模发展。此外, 阳极析氧反应存在较高的过电势, 从而导致当前制氢能耗与成本较高, 严重制约了其商业化应用。 为了解决上述问题与挑战,本文对当前发展较为成熟的碱性电解水技术进行了综合讨论与分析。 首先, 对电解水发展历程中的重要节点进行了总结, 便于读者了解该领域。进一步, 从电催化剂、 电极、 反应和系统的角度深入总结了提升电解水制氢性能的有效策略。作者分别介绍了近年来层状双金属氢氧化物基电解水催化剂、电解水制氢耦合氧化反应以及可再生能源驱动的电解水系统的重要研究进展; 同时对结构化催化剂在电解水应用中的构效关系进行了深入分析。最后, 对该领域存在的挑战和未来发展方向进行了展望,希望能为氢能的发展和推广提供一定的思路。
谢文富 , 邵明飞 . 碱性电解水高效制氢[J]. 电化学, 2022 , 28(10) : 2214008 . DOI: 10.13208/j.electrochem.2214008
Hydrogen production from water electrolysis is a sustainable and environmentally benign strategy in comparison with fossil fuel-based hydrogen. However, this promising technique suffers from the high energy consumption and unsatisfactory cost due to the sluggish kinetics of both half reaction and inferior stability of electrocatalysts. To address this challenge, herein, we present a timely and comprehensive review on advances in alkaline water electrolysis that is already commercialized for large scale hydrogen production. The design principles and strategies with aiming to promote the performance of hydrogen generation are discussed from the view of electrocatalyst, electrode, reaction and system. The challenges and related prospects are presented at last, hopefully to provide essential ideas and to promote the wide application of hydrogen production.

/
| 〈 |
|
〉 |