

低共熔溶剂辅助合成新型的网状纳米结构用于加速甲酸电氧化
收稿日期: 2022-06-23
修回日期: 2022-07-19
录用日期: 2023-07-21
网络出版日期: 2022-07-29
Deep Euteceic Solvents-Assisted Synthesis of Novel Network Nanostructures for Accelerating Formic Acid Electrooxidation
Received date: 2022-06-23
Revised date: 2022-07-19
Accepted date: 2023-07-21
Online published: 2022-07-29
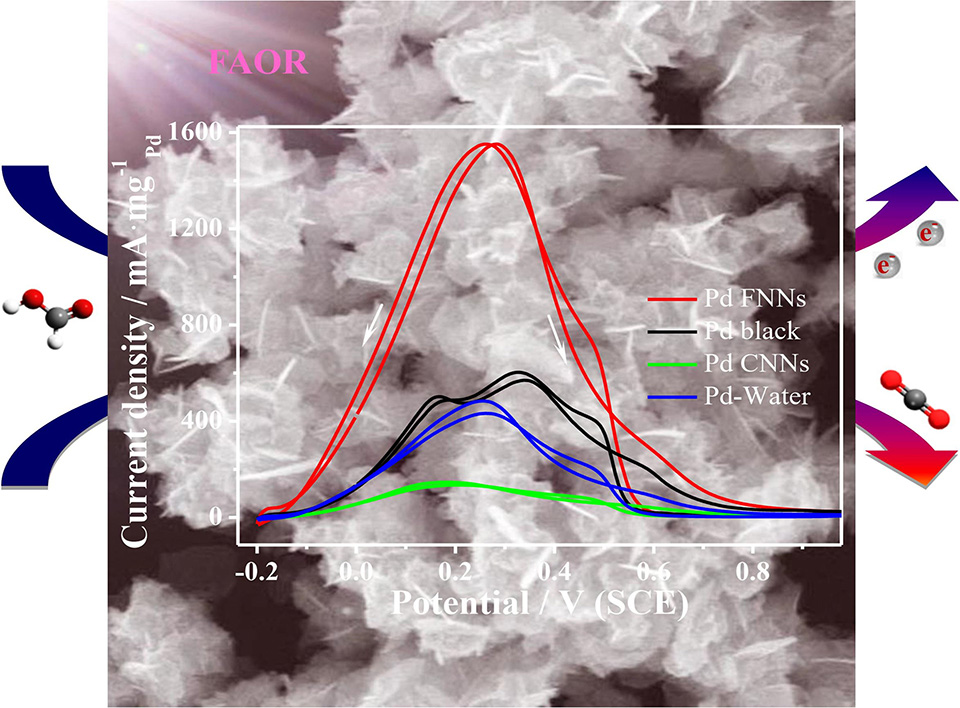
低共熔溶剂(DESs)是一种用于可控合成金属纳米结构的溶剂。在氯化胆碱-尿素DESs中,使用抗坏血酸作为还原剂可以制备由交错的纳米片和纳米球组成的花状Pd纳米颗粒,并且其自发地转化为三维网络纳米结构。此纳米网状结构的形成机制也有系统的研究,其中,DESs作为溶剂和软模板用于形成3D花状钯网络纳米结构(Pd-FNNs),CTAB和NaOH的用量在Pd-FNNs的各向异性生长和生成中起着至关重要的作用。Pd较低的电催化性能是阻碍燃料电池商业化应用的主要挑战之一。然而,具有较低表面能和丰富晶界的3D Pd-FNNs对甲酸氧化反应表现出增强的电催化活性和稳定性,其质量活性和本征活性分别是商业Pd黑催化剂的2.7和1.4倍。因此,此策略为合成独特的Pd基纳米结构提供了一种可行的路径。
张俊明 , 张小杰 , 陈瑶 , 房英健 , 樊友军 , 贾建峰 . 低共熔溶剂辅助合成新型的网状纳米结构用于加速甲酸电氧化[J]. 电化学, 2023 , 29(5) : 2206231 . DOI: 10.13208/j.electrochem.2206231
Deep eutectic solvents (DESs) have been reported as a type of solvent for the controllable synthesis of metal nanostructures. Interestingly, flower-like palladium (Pd) nanoparticles composed of staggered nanosheets and nanospheres are spontaneously transformed into three-dimensional (3D) network nanostructures in choline chloride-urea DESs using ascorbic acid as a reducing agent. Systematic studies have been carried out to explore the formation mechanism, in which DESs itself acts as a solvent and soft template for the formation of 3D flower-like network nanostructures (FNNs). The amounts of hexadecyl trimethyl ammonium bromide and sodium hydroxide also play a crucial role in the anisotropic growth and generation of Pd-FNNs. The low electrocatalytic performance of Pd is one of the major challenges hindering the commercial application of fuel cells. Whereas, the 3D Pd-FNNs with lower surface energy and abundant grain boundaries exhibited the enhanced electrocatalytic activity and stability toward formic acid oxidation, by which the mass activity and specific activity were 2.7 and 1.4 times higher than those of commercial Pd black catalyst, respectively. Therefore, the current strategy provides a feasible route for the synthesis of unique Pd-based nanostructures.

Key words: Deep eutectic solvents; Palladium; Network nanostructure; Formic acid
/
| 〈 |
|
〉 |