

一体式可再生燃料电池双功能氧催化剂的研究进展
收稿日期: 2022-05-30
修回日期: 2022-06-29
录用日期: 2022-07-14
网络出版日期: 2022-07-20
基金资助
国家自然科学基金(21978260);国家自然科学基金(22178307)
Recent Progress of Bifunctional Electrocatalysts for Oxygen Electrodes in Unitized Regenerative Fuel Cells
Received date: 2022-05-30
Revised date: 2022-06-29
Accepted date: 2022-07-14
Online published: 2022-07-20
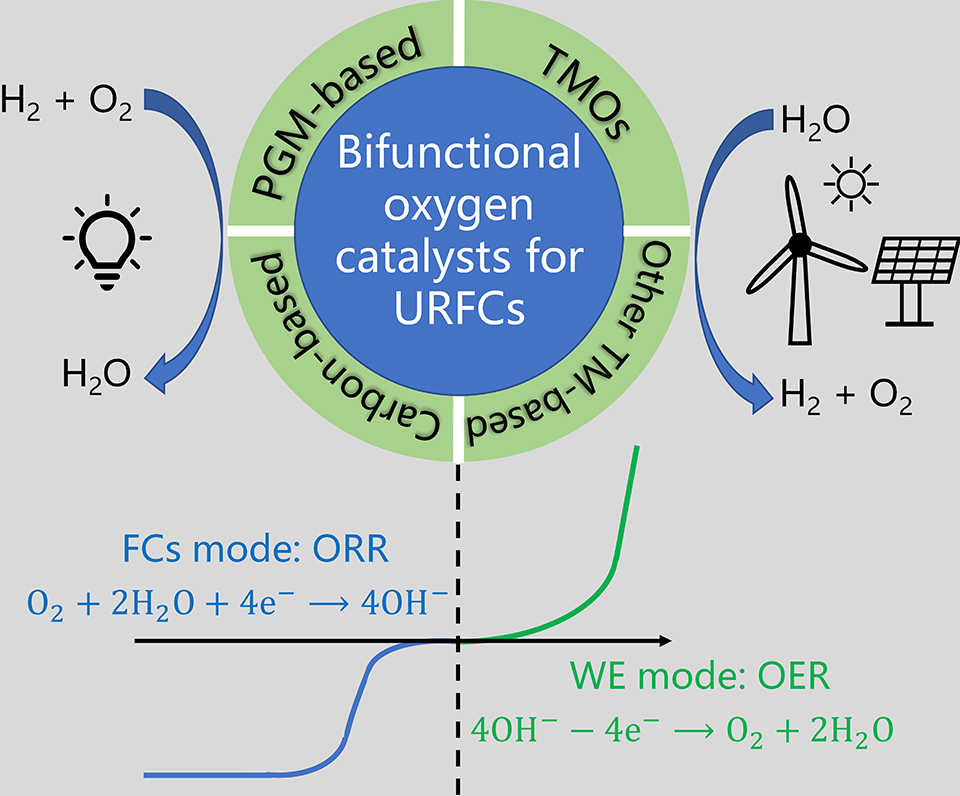
双功能氧催化剂的催化活性及稳定性是决定一体式可再生燃料电池能否高效运作的关键因素之一。得益于分别对于氧还原及氧析出反应特定中间产物适当的结合能,铂与铱、钌及其氧化物所制成的贵金属催化剂,常被应用于一体式可再生燃料电池中作为双功能氧催化剂。同时,近年来对于非铂族双功能氧催化剂的研究也取得了较大进展。本篇综述从一体式可再生燃料电池中氧还原及氧析出反应的作用机理出发,首先着重对传统铂基双功能催化剂的构效关系进行了总结,其次介绍了钙钛矿型、尖晶石型氧化物、非金属等新型双功能氧催化剂的发展趋势。此外,本文对于该研究领域所存在的限制条件和发展路线也进行了总结与展望。
郑天龙 , 欧明玉 , 徐松 , 毛信表 , 王释一 , 和庆钢 . 一体式可再生燃料电池双功能氧催化剂的研究进展[J]. 电化学, 2023 , 29(7) : 2205301 . DOI: 10.13208/j.electrochem.2205301
Unitized regenerative fuel cells (URFCs), which oxidize hydrogen to water to generate electrical power under the fuel cells (FCs) mode and electrolyze water to hydrogen under the water electrolysis (WE) mode for recycling, are known as clean and sustainable energy conversion devices. In contrast to the hydrogen oxidation reaction (HOR) and hydrogen evolution reaction (HER) on the hydrogen electrode side, the sluggish kinetics of oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) on the oxygen electrode side requires highly efficient bifunctional oxygen catalysts. Conventional precious metal catalysts combine Pt and IrO2 with excellent ORR and OER activities to achieve bifunctional electrocatalysis performance, but the scarcity and high cost of precious metals have restricted their applications. Although platinum group metal (PGM)-free bifunctional catalysts circumvent the problems of high price and scarce resources, they suffer from insufficient activity and poor stability. Therefore, much attention has been paid by researchers on developing efficient, durable and low-cost bifunctional oxygen catalysts. In this review, we mainly introduce the recent advances in bifunctional oxygen catalysts for URFCs focusing on the catalyst design, activity, and durability. First of all, the fundamental understanding of the ORR and OER mechanisms is essential prior to discussing the development of bifunctional oxygen catalysts. Starting from activity descriptor-based approaches in the identification of catalyst activity, this review summarizes the alternative catalyst design strategies confronted with the unfavorable scaling relationship existing among the binding energies of different oxygen-containing reaction intermediates during ORR and OER. Subsequently, in addition to introducing the design strategies of conventional PGM-based bifunctional catalysts, the recent progress of PGM-free bifunctional catalysts, including perovskite oxides, spinel oxides, other transition metal compounds, and carbon-based (non-metal) catalysts, is presented in terms of their structure-property relationship. Various strategies have been developed by researchers to optimize the performance of PGM-free bifunctional catalysts, such as nanostructuring, defects engineering, heteroatom doping, phase and composition modulation, support coupling and morphology engineering, etc. Some PGM-free bifunctional catalysts reported in the literature show promising ORR and OER activities superior to Pt+IrO2 in an alkaline environment. In general, although great progress has been made on PGM-free bifunctional electrocatalysts, their cycling durability is still far from that of precious metal catalysts, and few of them have been applied in acidic environments. Therefore, much more efforts are needed to improve the stability of PGM-free bifunctional catalysts. Lastly, the challenge and future development of designing optimal bifunctional oxygen catalysts are discussed.

/
| 〈 |
|
〉 |