

超微电极实验:基本原理、制备方法和伏安性能
收稿日期: 2022-07-08
修回日期: 2022-07-31
录用日期: 2022-12-02
网络出版日期: 2022-12-02
Ultramicroelectrode Experiments: Principles, Fabrications and Voltmmetric Behaviors
Received date: 2022-07-08
Revised date: 2022-07-31
Accepted date: 2022-12-02
Online published: 2022-12-02
马桢 , 林佳阳 , 南文静 , 韩联欢 , 詹东平 . 超微电极实验:基本原理、制备方法和伏安性能[J]. 电化学, 2023 , 29(7) : 2216002 . DOI: 10.13208/j.electrochem.2216002
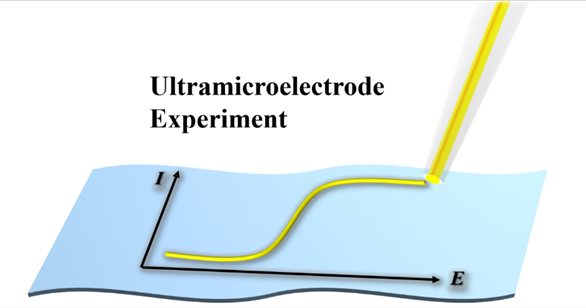
Due to the small size at least in one dimension (< 25 μm), ultramicroelectrode (UME) has small electric-double-layer capacitance, low IR drop, rapid mass transfer rate, fast response, high signal/noise ratio and high spatiotenporal resolution. UME is qualified not only to study the kinetics of fast electrode processes, but also to act as the probe of scanning electrochemical microscopies to obtain the localized chemical or electrochemical reactivity of the substrates. Thus, UMEs play a significant role in various research domains of electrochemistry, and have become an important electrochemical experimental method. Herein, we will introduce the basic principles, a simple fabrication method and voltammetric experimental protocols of UME, providing a guide to carry out the UME experiments.
/
| 〈 |
|
〉 |