

乙烯在钯圆盘电极的电化学氧化研究
Electrochemical Oxidation of Ethylene on Palladium Electrode
Received date: 2022-05-31
Revised date: 2022-06-27
Accepted date: 2022-07-12
Online published: 2022-07-13
由于巨大的潜在市场,乙烯的电化学氧化受到愈来愈多的关注。目前,主流的电化学氧化法仍以依赖于氧化还原媒介的介导氧化法为主,而这些媒介的使用在电解过程中产生大量的腐蚀性中间体,使其实际应用受到阻碍。直接电氧化法可有效规避此问题,但又受到低活性和低选择性的限制。在本工作中,我们针对目前最先进的钯催化直接氧化体系,在中性条件下开展了一系列电化学研究,以对该过程的机理获取更深入的认识。在氮气和乙烯氛围下,钯电极的循环伏安谱图有显著区别。我们发现电解过程中生成的Pd(II)物种在乙烯氛围下可绕过原本的电化学还原路径,通过一个化学步还原为Pd(0),因此可能是乙烯氧化的活性位点。Pd(II)物种所对应的还原峰也因此可作为乙烯吸附的数量的指标。通过电化学脉冲序列的设计,我们在钯催化剂上识别了两种具有不同吸附强度的乙烯,其强、弱吸附模式所对应的电荷转移比例约为0.3:1。弱吸附的乙烯在钯电极表面表现出可逆的吸脱附行为,而具有强吸附模式的乙烯无法通过物理过程脱附,可能指向到乙烯深度氧化过程。这项工作为进一步设计高性能乙烯直接电氧化催化剂提供了设计思路和方向。
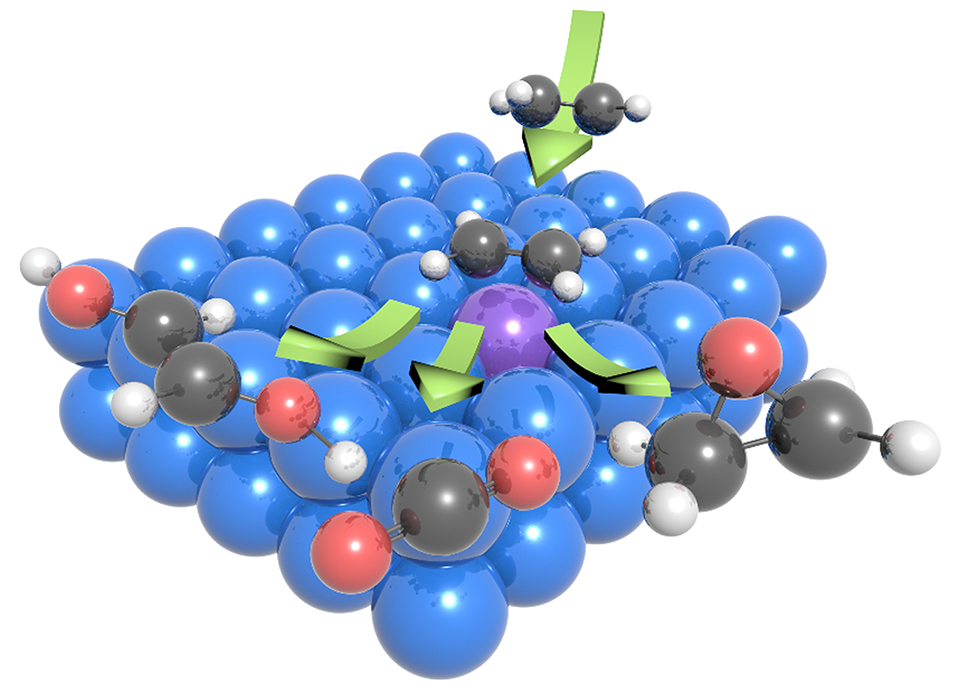
吴炜星 , 王莹 . 乙烯在钯圆盘电极的电化学氧化研究[J]. 电化学, 2023 , 29(1) : 2215004 . DOI: 10.13208/j.electrochem.2215004
The electrochemical oxidation of C2H4 is attracting increasing attention due to its vast potential market. The current electrochemical methods rely on the use of redox mediators, which may produce corrosive intermediates, while direct oxidation is still limited by its low activity and selectivity. Herein, we conducted electrochemical studies to obtain mechanistic insights into the benchmark Pd catalyst. The generated Pd(II) could be the active site for C2H4 oxidation. By designing the pulse sequence, we found the ratio of strongly and weakly adsorbed C2H4 on Pd to be 0.3:1. The result we obtained provides a guideline for the rational design of high-performance C2H4 oxidation catalysts.

/
| 〈 |
|
〉 |