

氧化钨和磷钨酸对LiNi0.96Co0.02Mn0.02O2材料的表面包覆改性研究
收稿日期: 2022-04-28
修回日期: 2022-05-17
录用日期: 2022-07-01
网络出版日期: 2022-07-05
基金资助
福厦泉自创区协同专项(3502ZCQXT202004)
Surface Modifications of LiNi0.96Co0.02Mn0.02O2 with Tungsten Oxide and Phosphotungstic Acid
Received date: 2022-04-28
Revised date: 2022-05-17
Accepted date: 2022-07-01
Online published: 2022-07-05
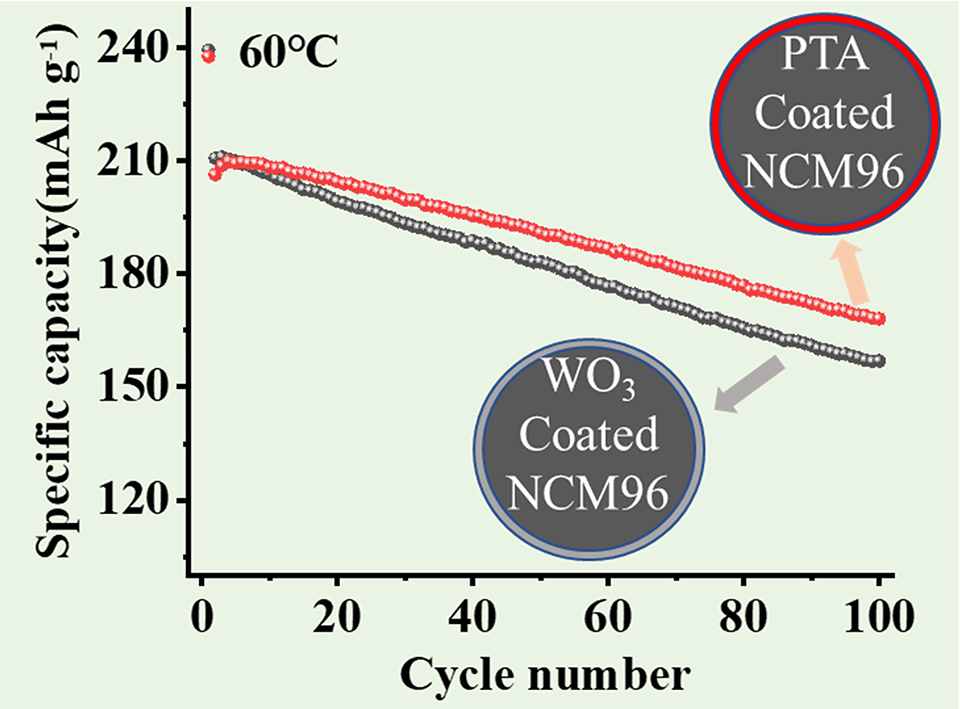
随着电动汽车的高速发展,对锂离子电池的能量密度、循环性能和成本提出了更高的要求,目前已有的高镍材料LiNi0.8Co0.1Mn0.1O2(NCM811)能量密度可以达到760 Wh·kg-1,已成为锂离子正极材料发展的重要方向。超高镍三元正极材料( LiNixCoyMn1-x-yO2,x ≥ 0.90)具有超过210 mAh·g-1比容量,因而可实现更高的能量密度,但目前关于超高镍材料的研究工作仍然比较少。超高镍正极材料的研究极具实用意义,因此,本文选择LiNi0.96Co0.02Mn0.02O2(NCM96)这一超高镍材料进行研究。为了提升超高镍三元正极材料NCM96的电化学性能,本工作采用了氧化钨和磷钨酸来对其进行包覆改性,并系统研究了材料改性前后的结构、形貌及电化学性能。其中,氧化钨包覆能有效提升三元材料的电化学性能,但目前尚未有利用氧化钨对超高镍正极材料进行包覆改性的报道。此外,磷钨酸是一种可以同时实现氧化钨和磷酸盐双重包覆的物质,双重包覆有望实现比单一元素包覆更优的电化学性能。本工作通过NCM96前驱体与磷钨酸和氧化钨液相共混,烘干后混锂烧结实现氧化钨和磷钨酸包覆。研究结果表明,两种表面改性方法对超高镍三元正极材料首圈放电比容量影响都较小,且能有效改善材料的长期循环性能。对比两种改性材料的高温电化学性能,发现经磷钨酸包覆改性后的材料其高温循环性能优于氧化钨包覆改性,说明磷钨酸的P/W双元素改性优于WO3的W单元素改性。
关键词: 锂离子电池; 超高镍低钴三元正极材料; 表面包覆; 氧化钨包覆; 磷钨酸包覆
赵刚 , 龚正良 , 李益孝 , 杨勇 . 氧化钨和磷钨酸对LiNi0.96Co0.02Mn0.02O2材料的表面包覆改性研究[J]. 电化学, 2023 , 29(10) : 2204281 . DOI: 10.13208/j.electrochem.2204281
With the rapid development of electric vehicles, enormous demands are made for higher energy density, better cycling performance and lower cost of lithium-ion batteries (LIBs). As an important high capacity cathode material for LIBs,the high nickel layered oxide material LiNi0.8Co0.1Mn0.1O2 (NCM811) can reach an energy density of 760 Wh·kg-1. The ultra-high nickel ternary positive electrode material (LiNi1-x-yCoxMnyO2, x ≥ 0.90) has a specific capacity of more than 210 mAh·g-1, and can realize higher energy density. Besides, an ultra-high nickel material uses lower cobalt content, and reduces material cost. Tungsten oxide coating has been reported to effectively improve the electrochemical performance of ternary materials, but no reports can be found for tungsten oxide coating modified ultra-high nickel cathode materials. On the other hand, phosphate coating has been widely used in surface coating modification of high nickel cathode materials to improve their electrochemical performance, but it is difficult to achieve uniform coating. Phosphotungstic acid (PTA) can function as a double coating with tungsten oxide (WO3) and phosphate at the same time, which is expected to achieve better electrochemical performance than single coating. In this work, LiNi0.96Co0.02Mn0.02O2 (NCM96) was selected. The NCM96 precursor and PTA/WO3 were dispersed in ethanol for mixing. After drying, the product was mixed with lithium source and sintered, so as to achieve tungsten oxide and phosphotungstic acid coating. The structures, morphologies and electrochemical performances of the PTA modified and WO3 modified NCM96 materials are compared. The results showed that, in the process of either PTA or WO3 coating modification, W and P elements were not doped into the lattice of NCM96 material, forming a relatively uniform coating structure, in which the WO3 Coating modification led to single element coating structure, while the PTA coating modification led to P/W double elements coating structure. Electrochemical test and analysis revealed that the two types of the surface modification methods had no effects on the first cycle discharge capacity of the NCM96 material, while had effectively improved the long-term cycling performances. By comparing the high temperature electrochemical performance of the WO3 and PTA coated samples, the PTA coated sample NCM96@1wt%PTA material exhibited superior cycling stability at 60 oC, indicating that the P/W double elemental surface modification with PTA is superior to the W single elemental modification with WO3.

/
| 〈 |
|
〉 |