

电解液中Cu(111)晶面电溶解/沉积势垒施加电荷相关性的跨尺度计算
收稿日期: 2022-05-17
修回日期: 2022-06-30
录用日期: 2022-07-02
网络出版日期: 2022-07-05
基金资助
国家自然科学基金项目(12072179);国家自然科学基金项目(11672168);云南省重大科技专项(202002AB080001);之江实验室科研攻关项目(2021PE0AC02)
Charge-Dependence of Dissolution/Deposition Energy Barrier on Cu(111) Electrode Surface by Multiscale Simulations
Received date: 2022-05-17
Revised date: 2022-06-30
Accepted date: 2022-07-02
Online published: 2022-07-05
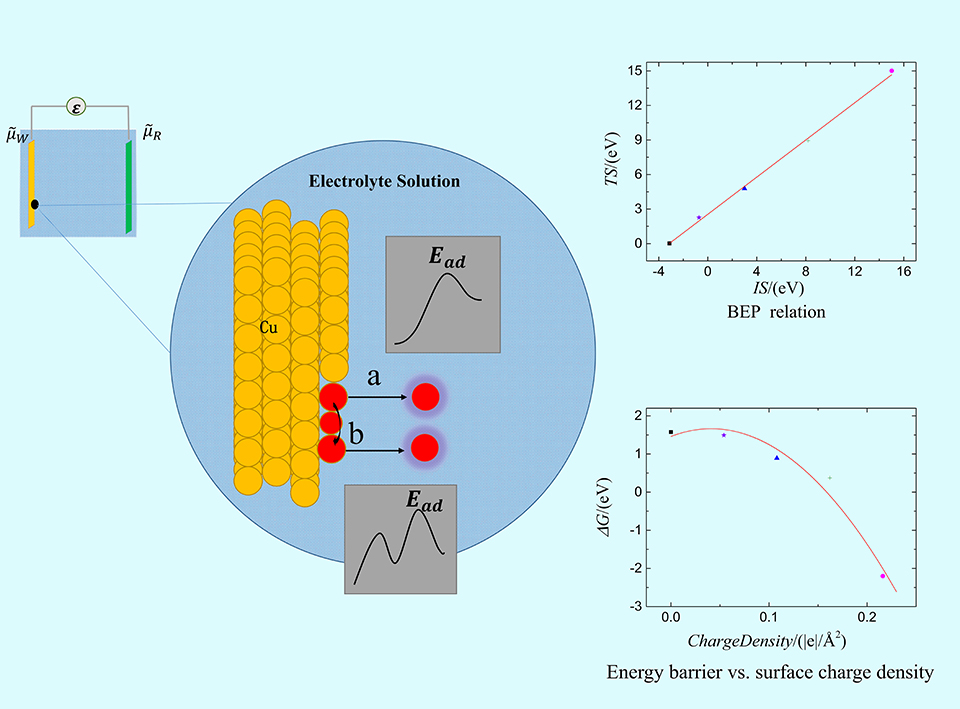
电化学沉积和电化学腐蚀的核心问题是不同电压/电荷作用下的电极/电解质界面行为,其控制量是溶解/沉积反应路径的势垒,但是势垒的测量和计算难度比较大。本文采用密度泛函和连续介质耦合方法研究了不同加载电荷面密度下平整表面和含阶梯表面的Cu(111)面薄板电极直接和间接溶解/沉积两种路径的能量形态。结果发现,不同加载电荷面密度下溶质Cu原子在Cu(111)面的表面扩散和溶解过程中初末态能量分别和最高过渡态能量存在简单的线性关系,符合经典的Brønsted-Evans-Polanyi关系。在直接/间接溶解和沉积过程中,势垒和加载的电荷面密度呈线性或二次函数关系。通过这些表达式可以直接从稳态能量计算溶解/沉积和表面扩散的势垒,也可以直接计算不同加载电荷面密度下的势垒,极大的降低实验和计算工作量。通过拟合公式计算出不同临界加载电荷面密度时的势垒大小可以得出:对于溶解过程中,随着加载电荷面密度逐渐增大至0.135 |e|/Å2,阶梯处原子首先以直接溶解的方式进入到电解质溶液中;对于沉积过程,随着加载电荷面密度降低至0.105 |e|/Å2,电沉积首先发生在平整表面,并可越过较低的表面扩散势垒移动至台阶处,表面扩散是速率控制步骤。当加载电荷面密度进一步减小为0.086 |e|/Å2,此时的沉积方式以直接沉积到阶梯位置为主。
乔行 , 朱勇 , 孙升 , 张统一 . 电解液中Cu(111)晶面电溶解/沉积势垒施加电荷相关性的跨尺度计算[J]. 电化学, 2023 , 29(10) : 2205171 . DOI: 10.13208/j.electrochem.2205171
Behaviors of electrified interface under different applied potentials/charges play the central role in electroplating process and electrochemical corrosion. The mechanism, however, is unclear yet for a surface atom dissolving/depositing from/on an electrode surface under an applied potential. The energy barrier along the reaction path is the key variable. The present work conducts hybrid first-principles/continuum calculations to study the direct and indirect dissolution/deposition of a Cu atom on perfect/stepped Cu(111) planar electrodes in an electrolyte under different excess charges. Energy profiles present a linear relationship between the energies of the initial/final state and the activation state of different reaction paths under different applied charges, obeying the Brønsted-Evans-Polanyi relation. The activation energy is also a linear or quadratic function of charge density per unit surface area during direct and indirect dissolution/deposition. These simple relations provide a simple way to deduce the activation energy from the energy of stable states under different charge levels. Analytical formula indicates the occurrence of automatic dissolution from step sites at the applied surface charge density larger than 0.135 |e|/Å2. When the applied charge density is between 0.086 |e|/Å2 and 0.105 |e|/Å2, the energy barrier for electrodeposition to the planar surface becomes smaller than zero, while there is a small barrier for surface diffusion, indicating indirect deposition with surface diffusion as the rate determining step. When the applied surface charge density further decreases to lower than 0.086 |e|/Å2, the concentration effects of the available deposition sites on steps and planar surface are ignored, becoming mainly the direct deposition because of the energy barrier of surface diffusion.

/
| 〈 |
|
〉 |