

Sn-Ag-Cu三元合金焊料电沉积中添加剂的影响研究
收稿日期: 2021-05-06
修回日期: 2022-05-30
网络出版日期: 2022-06-28
Study on the Effect of Additives in the Electrodeposition of Sn-Ag-Cu Ternary Alloy Solder
Received date: 2021-05-06
Revised date: 2022-05-30
Online published: 2022-06-28
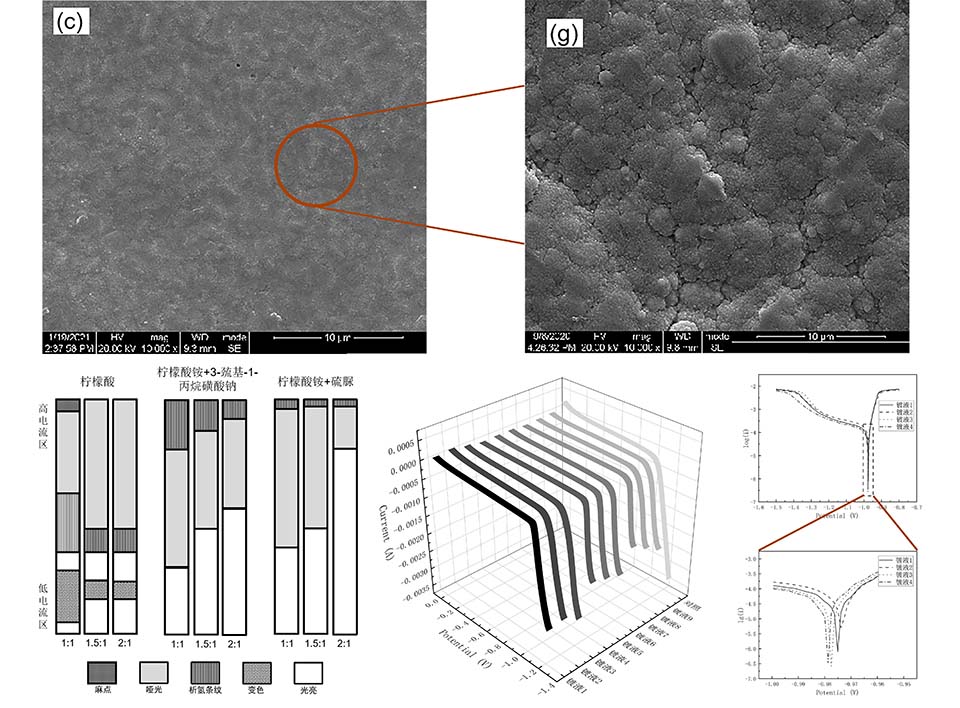
Sn-Ag-Cu三元合金是目前最为理想的Sn-Pb合金替代品,采用电沉积的方法来制备Sn-Ag-Cu三元合金,具有生产效率高、设备简单、维护方便、镀层性能优良等优点。通过霍尔槽实验确定了电镀液的配位体系;通过微观形貌表征、电化学腐蚀测试以及阴极极化曲线测试,确定了镀液中使用的光亮剂种类及二者的比例;通过微观形貌表征的方法,确定了电镀液中的分散剂;通过测定镀液中Sn2+离子的浓度变化,确定了镀液中的稳定剂。结果表明,当使用柠檬酸铵和硫脲作为配位剂,光亮剂选用苄叉丙酮和聚乙二醇,分散剂选用聚丙二醇,稳定剂选用抗坏血酸时,镀液阴极极化作用强,稳定性强,镀层致密平整,抗腐蚀能力强。
缪桦 , 李明瑞 , 邹文中 , 周国云 , 王守绪 , 叶晓菁 , 朱凯 . Sn-Ag-Cu三元合金焊料电沉积中添加剂的影响研究[J]. 电化学, 2022 , 28(6) : 2104411 . DOI: 10.13208/j.electrochem.210441
Sn-Ag-Cu ternary alloy is the most ideal substitute for Sn-Pb alloy at present, and can be prepared by electrodeposition with high production efficiency, simple equipment, easy maintenance and excellent coating performance. The coordination system of the electroplating solution was investigated through the Hull cell experiment. The type of brighteners used in the plating solution and the ratio of the two brighteners were determined through the microscopic morphologic characterization, electrochemical corrosion test and cathodic polarization curve test. In addition, the dispersant and stabilizer were studied by observing the microscopic morphology and measuring the concentration change of Sn2+ ions in the plating solution, respectively. The results showed that when ammonium citrate and thiourea were used as the complexing agents, benzylidene acetone and polyethylene glycol as the brighteners, poly propylene glycol as the dispersing agent, and ascorbic acid as the stabilizer, the wide current density range of the plating solution and excellent stability were obtained. Furthermore, the resulted coating was dense and smooth with high corrosion resistance.

Key words: ternary alloy; electrodeposition; additive; Sn-Ag-Cu
/
| 〈 |
|
〉 |