

吸电子和亲水性Co-卟啉促进电催化氧还原反应的研究
收稿日期: 2022-04-13
修回日期: 2022-05-17
网络出版日期: 2022-05-25
A Co Porphyrin with Electron-Withdrawing and Hydrophilic Substituents for Improved Electrocatalytic Oxygen Reduction
# These authors contributed equally to this work.
Received date: 2022-04-13
Revised date: 2022-05-17
Online published: 2022-05-25
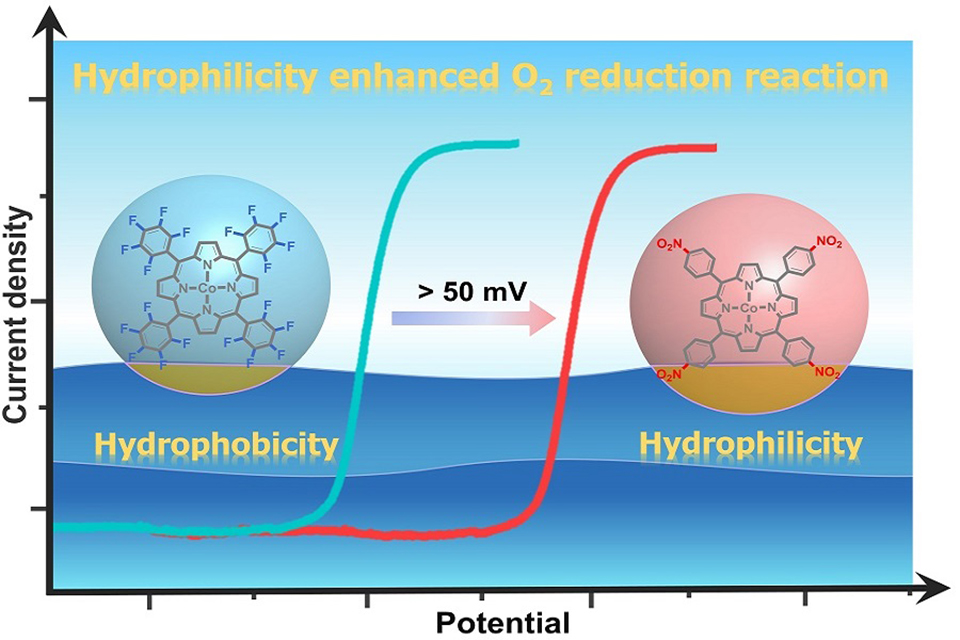
研究影响电催化氧还原反应活性的因素对于合理设计高效的氧还原反应催化剂至关重要。调节催化剂电子结构通常被用于精确调控电催化氧还原反应活性。然而, 该反应发生在液/气/固界面, 很少有报道调控分子催化剂的亲疏水性来提高其催化活性。在此, 我们报道了两种钴卟啉NO2-CoP(5,10,15,20-四(4-硝基苯基)钴卟啉)和5F-CoP(5,10,15,20-四(五氟苯基)钴卟啉)并研究了其电催化氧还原反应性能。通过同时调控meso-位取代基的电子结构和亲水性能, NO2-CoP显示出比5F-CoP更高的电催化氧还原反应活性, 其半波电位向阳极方向移动近60 mV。NO2-CoP比5F-CoP具有更好的亲水性。理论计算表明, NO2-CoP比5F-CoP更容易有效地与O2分子结合形成CoIII-O2·-。这项工作提供了一个简单而有效的策略, 通过使用吸电子和亲水取代基来提高钴卟啉的氧还原反应活性。该策略对于设计和开发其他用于电催化的分子催化剂体系也具有重要的启发意义。
郭鸿波 , 王亚妮 , 郭凯 , 雷海涛 , 梁作中 , 张学鹏 , 曹睿 . 吸电子和亲水性Co-卟啉促进电催化氧还原反应的研究[J]. 电化学, 2022 , 28(9) : 2214002 . DOI: 10.13208/j.electrochem.2214002
Understanding factors that influence the catalyst activity for oxygen reduction reaction (ORR) is essential for the rational design of efficient ORR catalysts. Regulating catalyst electronic structure is commonly used to fine-tune electrocatalytic ORR activity. However, modifying the hydrophilicity of catalysts has been rarely reported to improve ORR, which happens at the liquid/gas/solid interface. Herein, we report on two Co porphyrins, namely, NO2-CoP (Co complex of 5,10,15,20-tetrakis(4-nitrophenyl)porphyrin) and 5F-CoP (Co complex of 5,10,15,20-tetrakis(pentafluorophenyl)porphyrin), and their electrocatalytic ORR features. By simultaneously controlling the electronic structure and hydrophilic property of the meso-substituents, the NO2-CoP showed higher electrocatalytic activity than the 5F-CoP by shifting the ORR half-wave potential to the anodic direction by 60 mV. Compared with the 5F-CoP, the complex NO2-CoP was more hydrophilic. Theoretical calculations suggest that NO2-CoP is also more efficient than 5F-CoP to bind with an O2 molecule to form CoIII-O2·-. This work provides a simple but an effective strategy to improve ORR activity of Co porphyrins by using electron-withdrawing and hydrophilic substituents. This strategy will be also valuable for the design of other ORR molecular electrocatalysts.

/
| 〈 |
|
〉 |