

多孔陶瓷支撑型管式固体氧化物电解池性能研究
收稿日期: 2022-04-13
修回日期: 2022-05-14
录用日期: 2022-05-17
网络出版日期: 2022-05-18
基金资助
国家自然科学基金(22005051);国家自然科学基金(51872047);广东省基础与应用基础研究基金(2022A1515012001);广东省基础与应用基础研究基金(2019A1515110237);广东省普通高校科研项目(2019KQNCX166);广东省普通高校科研项目(2019KZDXM039);及佛山市高校教师特色创新研究项目(2020XCC09)
Electrochemical Performance of Porous Ceramic Supported Tubular Solid Oxide Electrolysis Cell
Received date: 2022-04-13
Revised date: 2022-05-14
Accepted date: 2022-05-17
Online published: 2022-05-18
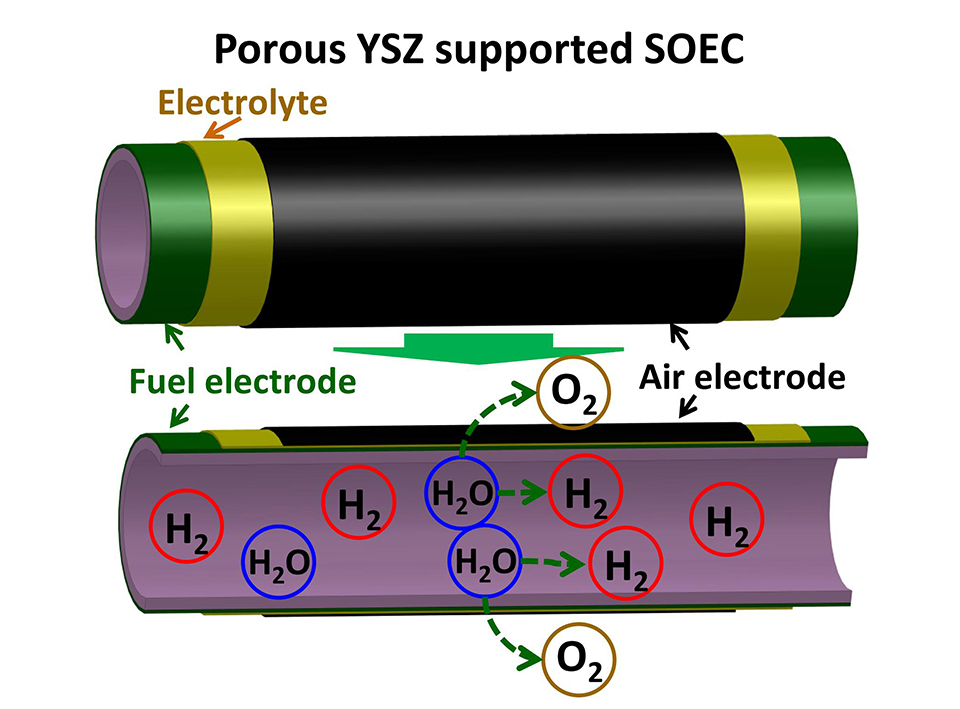
固体氧化物电解池是一种新型能源转换技术,能实现间歇式能源到氢能的高效转化,为能源的有效利用提供了新途径。本文针对固体氧化物电解池金属镍基阴极支撑体在电解过程中的局部氧化以及由此引发的电池结构稳定性问题,提出了一种多孔氧化钇稳定的二氧化锆(YSZ)支撑型管式固体氧化物电解池,其构型为多孔YSZ支撑体/Ni-YSZ燃料极电流收集层/Ni-YSZ燃料极电化学催化层/YSZ/Ce0.8Sm0.2O1.9双层电解质层以及La0.6Sr0.4Co0.2Fe0.8O3-δ空气电极,研究了造孔剂(聚甲基丙烯酸甲酯,PMMA)的含量对多孔YSZ支撑体的孔隙率、孔径分布和支撑体机械强度的影响,考察了电解池在H2O-H2气氛中的电化学电解性能。研究结果表明,当PMMA含量为25wt.%时,电解池具有最优的综合力学性能和电解催化活性,在750 °C的工作温度下,电解池的产氢气速率为3 mL·min-1·cm-2,电解池在10次升降温热循环过程中电解性能衰减为~5%,表现出优良的电解稳定性。本研究结果验证了多孔YSZ支撑型管式电解池的应用可行性。
汪恒吉 , 陈文国 , 全周益 , 赵凯 , 孙毅飞 , 陈旻 , 奥坚科·弗拉基米尔 . 多孔陶瓷支撑型管式固体氧化物电解池性能研究[J]. 电化学, 2023 , 29(12) : 2204131 . DOI: 10.13208/j.electrochem.2204131
Solid oxide electrolysis cell (SOEC) is an efficient and clean energy conversion technology that can utilize electricity obtained from renewable resources, such as solar, wind, and geothermal energy to electrolyze water and produce hydrogen. The conversion of abundant intermittent energy to hydrogen energy would facilitate the efficient utilization of energy resources. SOEC is an all-ceramic electrochemical cell that operates in the intermediate to high temperature range of 500-750 °C. Compared with traditional low temperature electrolysis technology (e.g., alkaline or proton exchange membrane cells operating at ~100 °C), the high-temperature SOEC can increase the electrolysis efficiency from 80% to ~100%, providing a new way for energy saving.
The SOEC single cells with the nickel (Ni)-yttira-stabilized zirconia (YSZ) fuel electrode supported configuration have received most intensive research effort. This is due to the high catalytic activity and electronic conductivity of Ni, as well as good oxygen ionic conductivity of YSZ, promoting the electrochemical reduction of steam in fuel electrode. However, under the high steam partial pressures, the Ni in the electrode could be occasionally oxidized NiO at the high operation temperature, leading to volume expansion of the supporting layer. This phenomenon would induce internal stress in cell functional layers, resulting in cracking or even failure of the single cell.
To address the above mentioned issues, we propose a porous YSZ supported tubular single cell with a configuration of porous YSZ support, Ni-YSZ fuel electrode current collector, Ni-YSZ fuel electrode electrochemical functional layer, YSZ/Ce0.8Sm0.2O1.9 bi-layer electrolyte, and La0.6Sr0.4Co0.2Fe0.8O3-δ air electrode. As the porous YSZ substrate exhibits high chemical and structural stabilities in a wide range of oxygen and steam partial pressures under the SOEC operating conditions, employing the porous YSZ as the single cell support is expected to improve mechanical stability of the whole single cell. In this work, the porous YSZ supported tubular electrolysis cell has been fabricated by extrusion and dip-coating technique. The porosity, pore size and mechanical property of the YSZ support were investigated with respect to the amount of polymethyl methacrylate (PMMA) pore former. At the PMMA amount of 25wt.%, the porous YSZ support showed the optimum porosity of 40%-45% and good bending strength of ~20 MPa. Electrochemical performance of the single cell for steam electrolysis has been characterized under the H2O-H2 co-feeding condition. At the operation temperature of 750 °C, the H2 production rate reached 3 mL·min-1·cm-2 and the cell maintained 95% of its initial performance during 10 thermal cycles, demonstrating the feasibility of the novel porous YSZ supported tubular cell design for solid oxide electrolysis cell.

/
| 〈 |
|
〉 |