

氮掺杂石墨毡对水系醌基氧化还原液流电池性能的影响
收稿日期: 2022-03-23
修回日期: 2022-05-04
录用日期: 2022-05-05
网络出版日期: 2022-05-09
基金资助
国家自然科学基金项目(51873007)
Nitrogen-Doped Graphite Felt on the Performance of Aqueous Quinone-Based Redox Flow Batteries
Received date: 2022-03-23
Revised date: 2022-05-04
Accepted date: 2022-05-05
Online published: 2022-05-09
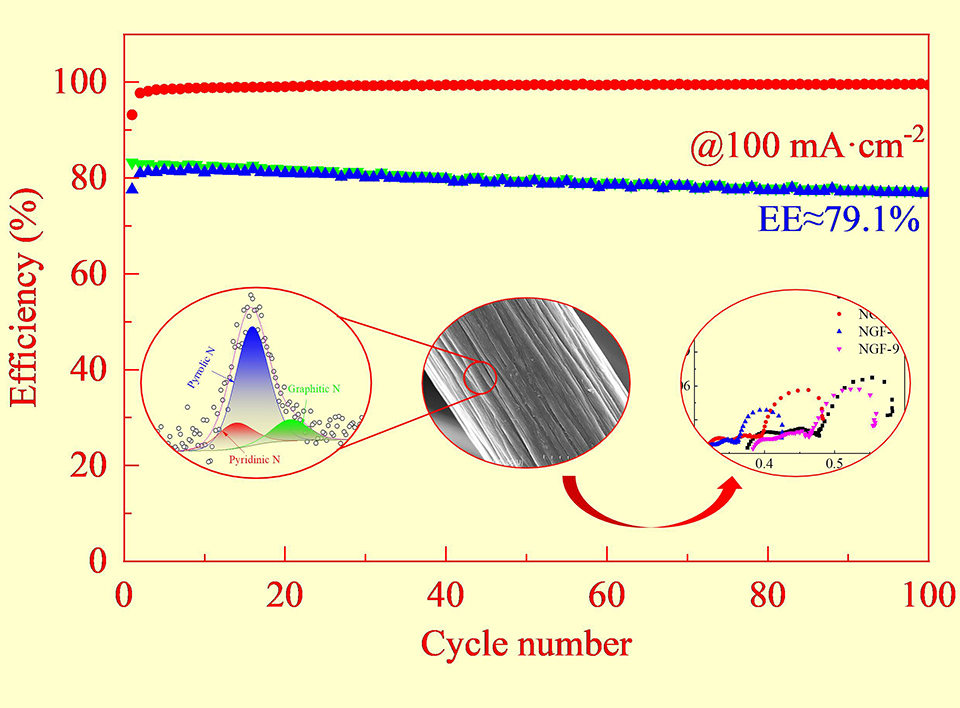
电极的性能是实现水系醌基氧化还原液流电池(AQRFBs)高能量效率的关键。本文采用尿素水热反应对石墨毡(GF)进行改性,同时研究了水热反应时间对氮掺杂石墨毡表面官能团和结构的影响。利用扫描电子显微镜(SEM)、比表面积及孔隙度分析仪(BET)、拉曼光谱(Raman)和X射线光电子能谱(XPS)对改性电极的表面形貌、比表面积、碳缺陷、元素含量和表面官能团进行了表征。然后,通过循环伏安法、电化学阻抗谱和单电池循环对改性电极的电化学性能进行了研究。结果表明,氮掺杂提高了石墨毡的比表面积、亲水性和电导率。氮掺杂石墨毡(NGFs)具有优异的电化学催化活性和较低的电荷转移电阻。与GF相比,在100 mA·cm-2时,电池负极使用NGF-6电极后,醌基氧化还原液流电池的能量效率提高了8.0%。
关键词: 氮掺杂石墨毡; 能量效率; 水系醌基氧化还原液流电池; 电荷转移电阻; 亲水性
张衡 , 夏力行 , 姜珊 , 王福芝 , 谭占鳌 . 氮掺杂石墨毡对水系醌基氧化还原液流电池性能的影响[J]. 电化学, 2023 , 29(12) : 2203231 . DOI: 10.13208/j.electrochem.2203231
Modification of electrode is vitally important for achieving high energy efficiency in aqueous quinone-based redox flow batteries (AQRFBs). The modification of graphite felt (GF) was carried out by means of urea hydrothermal reaction, and simultaneously, the effects of hydrothermal reaction time on the functional groups and surface structure of nitrogen-doped graphite felt were studied. The surface morphology and defect, element content and surface chemical state of the modified electrode were characterized by scanning electron microscopy (SEM), Brunauer-Emmett-Teller (BET) test, Raman spectroscopy, and X-ray photoelectron spectroscopy (XPS). The electrochemical performance of the modified electrodes was evaluated by cyclic voltammetry, electrochemical impedance spectroscopy and single cell test. These results indicate that the specific surface area, hydrophilicity and conductivity of GF have been improved by nitrogen doping. The nitrogen-doped graphite felt (NGF) demonstrates an outstanding electrochemical catalytic activity and less charge transfer resistance. With the NGF, the battery exhibited 8.0% increase in the energy efficiency of aqueous quinone redox flow batteries at 100 mA·cm-2.

/
| 〈 |
|
〉 |