

醇硫基丙烷磺酸钠对电解高性能锂电铜箔的影响
收稿日期: 2022-01-14
修回日期: 2022-02-21
网络出版日期: 2022-04-24
基金资助
常州市国际交流基金项目(CZ20200038);国家自然科学基金项目(51874050)
Effect of Sodium Alcohol Thiyl Propane Sulfonate on Electrolysis of High Performance Copper Foil for Lithium Ion Batteries
Received date: 2022-01-14
Revised date: 2022-02-21
Online published: 2022-04-24
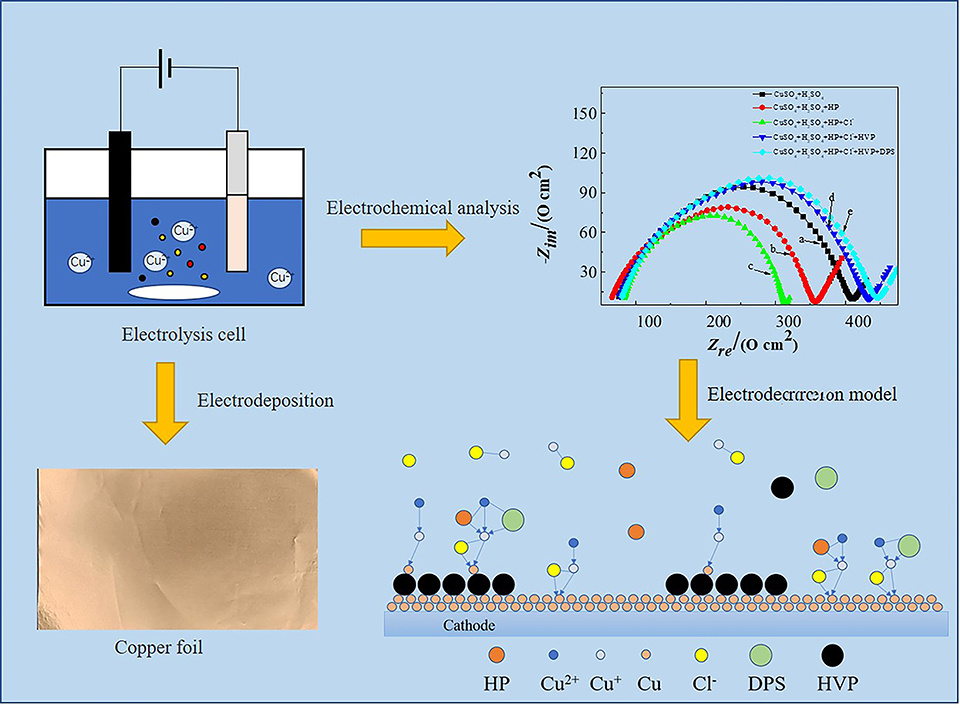
电解铜箔因其工艺简单、经济价值高,已广泛应用于印制线路板和锂离子电池领域。研究表明在电解制箔过程中,加入微量添加剂即可大幅度提高电解铜箔性能。因此, 在基础电解液(312.5 g·L-1 CuSO4·5H2O,100 g·L-1 H2SO4, 50 mg·L-1 Cl-)基础上,加入添加剂考察了电解液的电化学行为以及对铜箔表面形貌、结构以及性能的影响。实验选取了醇硫基丙烷磺酸钠(HP)、 水解蛋白(HVP)和N,N-二甲基硫代甲酰胺丙烷磺酸钠(DPS)作为组合添加剂, 利用扫描电镜(SEM)、 X射线洐射(XRD)以及电化学分析等方法,重点考察了组合添加中HP对铜箔表面形貌和物理性能的影响。研究结果表明,在组合添加剂体系中HP具有较强的去极化作用,可以加速铜核的生长,且具有增强铜(200)晶面的择优生长取向。HP与DPS、 HVP的协同作用可以进一步减小电解铜箔的晶粒尺寸,降低表面粗糙,提高铜箔力学性能和耐腐蚀性能。所制备的电解铜箔均匀致密,平均晶粒尺寸为29.2 nm、 平均粗糙度为 1.12 μm、 平均抗拉强度为399.5 MPa且耐蚀性能优越, 是锂离子电池负极集流体的理想材料, 具有较高的商业价值。
杨森 , 王文昌 , 张然 , 秦水平 , 吴敏娴 , 光崎尚利 , 陈智栋 . 醇硫基丙烷磺酸钠对电解高性能锂电铜箔的影响[J]. 电化学, 2022 , 28(6) : 2104501 . DOI: 10.13208/j.electrochem.210450
Electrolytic copper foils have been widely used in printed circuit boards and lithium-ion batteries due to their simple production process and high economic value. In the process of electrolysis foil making, additives can greatly improve the performance of electrolytic copper foils. In this work, the copper foils were prepared in a self-designed plate electrodeposition device of which the operating principles were in accordance with those of actual industrial production. A series of the Virgin Make-up Solution (VMS: 312.5 g·L-1 CuSO4·5H2O, 100 g·L-1 H2SO4, 50 mg·L-1 Cl-) containing different additives was investigated to study the electrochemical behaviors of the electrolytes and their effects on the surface morphology, structure, and properties of the electrolytic copper foils. The results showed that HP had a strong depolarization effect in the combined additive system, which can accelerate the growth of copper nuclei, and had the optimal growth orientation of the enhanced copper (200) crystal surface. HVP had adsorption effect on the cathode surface and formed a barrier layer on the cathode active site, which inhibits the electrical deposition of copper. DPS had a strong depolarization effect at low concentration, with the high concentration, a polarization effect reduced the grain size. When HP and DPS coexisted, there was a competitive adsorption, showing certain polarization effect. The synergistic effect of HP with DPS and HVP could further reduce the grain size of electrolytic copper foils, reduce the surface roughness, and improve the mechanical properties and corrosion resistance of the coatings. The obtained electrolytic copper foils were uniformly dense, with an average grain size of 29.2 nm, an average roughness of 1.12 μm. and an average tensile strength of 399.5 MPa. The electrolytic copper foils obtained exhibited the superior corrosion resistance, became the ideal materials for lithium-ion battery anode fluid collection, and had high commercial value. Subsequently, the effects of DPS and HVP in the combined additive system on the surface morphology and physical properties of copper foil will be investigated to further explore the action mechanism of the combined additive and improve the electrodeposition model.

/
| 〈 |
|
〉 |