

磷掺杂的Ru-Pt合金催化剂及其电催化碱性析氢性能
收稿日期: 2022-03-08
修回日期: 2022-03-22
录用日期: 2022-04-05
网络出版日期: 2022-04-07
P-doped Ru-Pt Alloy Catalyst toward High Performance Alkaline Hydrogen Evolution Reaction
Received date: 2022-03-08
Revised date: 2022-03-22
Accepted date: 2022-04-05
Online published: 2022-04-07
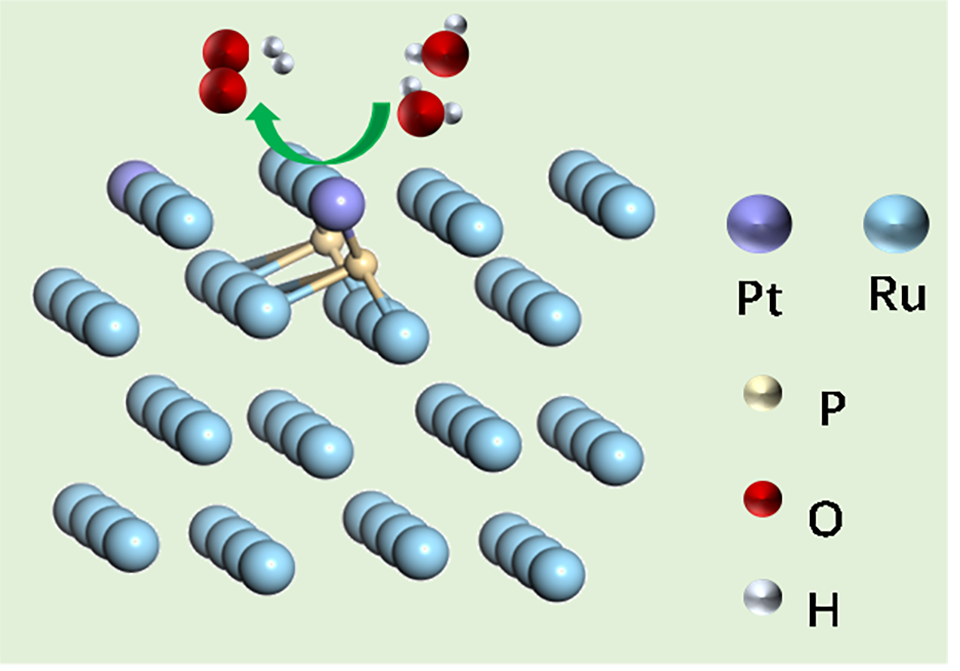
可再生能源驱动电催化水分解产氢气在现代氢能及氢燃料电池可持续发展方面,有着极其重要的地位。其中,性能优良催化剂的设计与开发又是重中之重。本文重点发展了一种磷掺杂的铂-钌合催化剂(Ru-P)#Pt/C),TEM分析确认Ru金属纳米粒子的球形形态,XRD表征Ru纳米粒子以六方密堆积形式存在。XPS分析进一步说明了Ru以金属态存在,Pt的原子比在14.5%左右,且以轻微氧化的状态存在,表明其可能与P成键。(Ru-P)@Pt合金催化剂在碱性电解液中表现出优异的电解水析氢性能,在10 mA·cm-2的电流密度下的过电位仅为17 mV vs. RHE,Tafel斜率值为27 mV·dec-1,表明该催化剂析氢决速步为Tafel步骤。而同等条件下,仅P掺杂的Ru催化剂及Pt负载的P掺杂的CNT,其性能均远逊于该目标催化剂,表明了P与Pt共掺杂的协同作用。(Ru-P)#Pt/C合金催化剂经过24 h耐久性测试,其10 mA·cm-2的过电位及稳定性测试后LSV电流仅出现轻微衰退。这表明P掺杂的Ru-Pt合金催化剂中Ru、Pt、P活性位点间的协同作用,显著提高了电催化析氢活性与稳定性,为高性能碱性电解水析氢催化剂的设计打开了广阔的前景。
黄荣钦 , 廖卫平 , 晏梦璇 , 刘石 , 李远明 , 康雄武 . 磷掺杂的Ru-Pt合金催化剂及其电催化碱性析氢性能[J]. 电化学, 2023 , 29(5) : 2203081 . DOI: 10.13208/j.electrochem.2203081
Electrocatalytic water splitting represents grand promise for hydrogen fuel in modern energy equipment, and the design and fabrication of higher performance catalysts are at the central. Herein, we report the sequential phosphorus (P)-doping into ruthenium (Ru) nanoparticles (Ru-P/C) by thermal annealing of Ru nanoparticles in phosphine (PH3) atmosphere and deposition of extremely low concentration of platinum (Pt) to obtain P-doped Ru-Pt alloy catalyst supported on carbon nanotubes (CNTs), which is denoted as (Ru-P)#Pt/C. The data by X-ray diffraction spectroscopy and transmission electron microscopy show that the Ru nanoparticles existed in the form of hexagonal close-packed (hcp) phase with low crystallinity. The results by high-resolution X-ray photoelectron spectroscopy indicate that Ru was mainly in metallic state, and Pt was slightly and positively charged, ascribing to the bonding with P atoms. This indicates that the highly diluted Pt atoms may be dispersed on the surface of Ru nanoparticles through Ru-P-Pt bonds. Accordingly, the as-prepared (Ru-P)#Pt/C alloy catalysts displayed excellent alkaline hydrogen evolution activity, revealing only 17 mV vs. RHE at a current density of 10 mA·cm-2 and a Tafel slope value of 27 mV·dec-1, superior to those of the controlled samples Ru-P/C and trace amount of Pt loaded P-doped CNTs (Pt/C-P). Density functional theory (DFT) calculation suggests that P-doping into Ru can enhance the adsorption of water molecules and the activation for water splitting, while the Pt site on Ru-Pt alloy can behave as the hydrogen desorption site. Thus, the superior performance of (Ru-P)#Pt/C alloy catalyst might be attributed to the synergistic effect of P-doped Ru sites and Pt sites, which significantly improves the alkaline hydrogen evolution reaction kinetics.

Key words: Ru-Pt alloy; Phosphorus-doping; Synergistic effect; Dual active sites
/
| 〈 |
|
〉 |