

孔雀石绿对金属钴超填充和成核过程的影响
收稿日期: 2021-03-25
修回日期: 2021-04-01
网络出版日期: 2022-04-02
基金资助
国家自然科学基金项目(21972037);城市水资源和和环境重点实验室(哈尔滨工业大学)(2021TS07)
Influences of Suppressing Additive Malachite Green on Superconformal Cobalt filling and Nucleation
Received date: 2021-03-25
Revised date: 2021-04-01
Online published: 2022-04-02
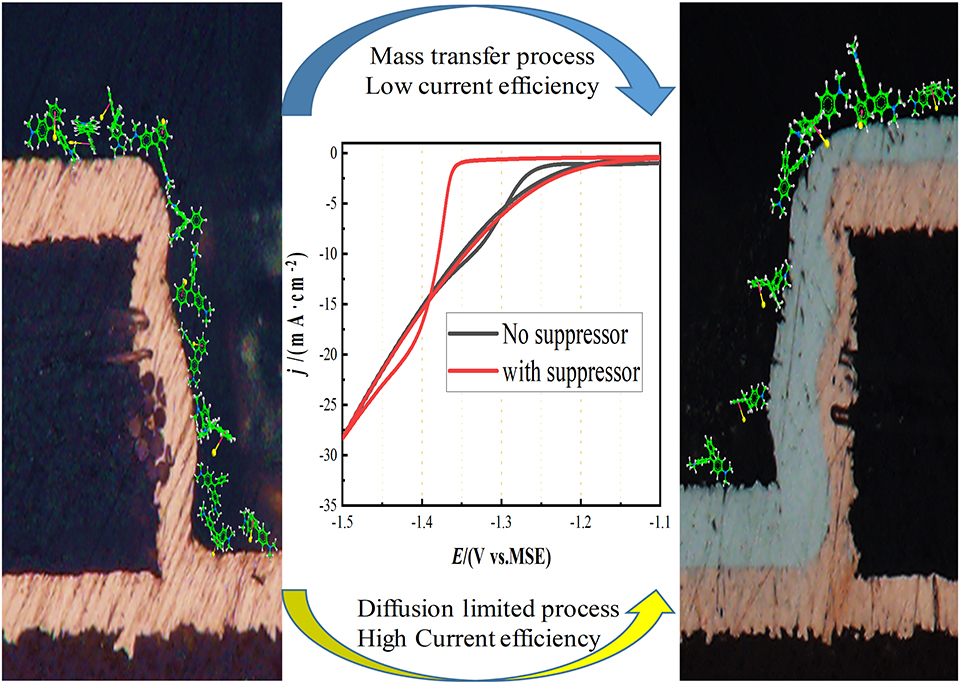
随着芯片制程低于7 nm,互连线后端填充的铜线电阻急剧增加,而平均自由程更低的金属钴(Co)可以用来取代铜,以减少由外表面和晶界处发生的散射导致的线电阻增长。在此选用硫酸钴(CoSO4)作为主盐,硼酸为缓冲剂,以孔雀石绿(MG)为抑制剂进行研究。通过电化学伏安法测试,发现随着MG浓度的增加,金属Co的沉积过电势逐渐增加,沉积受到抑制。利用电化学石英晶体微天平(EQCM)测试得出,MG的加入对整个沉积过程产生明显的抑制作用。这是因为MG容易吸附在阴极表面,与Co2+形成配位键,从而抑制了Co2+还原。随着对流过程的增强,阴极电流密度逐渐减小。最终确定镀液配方为0.4 mol·L-1 CoSO4, 0.5 mol·L-1硼酸, 少量Cl-, 20 mg·L-1 3-巯基-1-丙磺酸钠盐和10 mg·L-1 MG,在-1.27 V, pH = 4的条件下,可以实现微米级别PCB盲孔的超填充。由计时电流法测定的曲线分析得出金属Co的成核方式为三维瞬时成核。通过量化计算和分子静电势可知,静电势分布在35 ~ 78 kcal·mol-1 之间, MG分子中与氮相连的共轭结构吸附在阴极表面,而其中的苯环结构通过离域π键结构与Co2+发生作用,从而抑制了Co2+沉积过程。
马晓川 , 李亚强 , 杨培霞 , 张锦秋 , 安茂忠 . 孔雀石绿对金属钴超填充和成核过程的影响[J]. 电化学, 2022 , 28(6) : 2104521 . DOI: 10.13208/j.electrochem.210452
As the semiconductor integrated circuits evolve into 7 nm technology and beyond, the resistance of the Cu filling at back-end-of-line interconnects no longer linearly scales with dimension. The metal Co with lower mean free path can be used to replace Cu for reducing the line resistance caused by the scattering on the outer surface and grain boundary in the smaller and smaller size. In this study, CoSO4 was used as the main salt, boric acid as a buffer and malachite green (MG) as an inhibitor for further research. According to the electrochemical cyclic voltammetric (CV) curves by using a rotating disk electrode, the reductive overpotential of the metal Co shifted negatively and the reduction process was gradually suppressed with the increasing concentration of the additive MG. Besides, the deposition current density decreased and the current efficiency of the reduction process declined after adding MG from the electrochemical quartz crystal microbalances (EQCM) test. This is because of the MG adsorption on the surface and complexation with the metal Co ion in the bath. Hence, MG has a significant inhibition effect in the deposition process, which reduces the deposition efficiency. At -1.27 V, the current density of the total reduction process gradually decreased with the applying higher rotation rate and returned to the initial value within a few minutes after suspending. The current density was heavily influenced by the convection process. At last, the bottom-up superfilling of blind via from an aqueous solution containing 0.4 mol·L-1 CoSO4, 0.5 mol·L-1 H3BO3, a small amount of Cl-, 20 mg·L-1 MPS and 10 mg·L-1 MG at -1.27 V with the pH 4. From the chronoamperometric curves, the appearance of two peaks was mainly attributed to the hydrogen evolution process and the nucleation process of Co2+ reduction, and the nucleation process was three-dimensional instantaneous nucleation process. In order to further studying the construction of the MG, and the bonding between additive MG and cobalt ions, the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) of MG are obtained by quantum chemical calculation, indicating the active site distributed on the conjugate structure of aniline and amido cyclohexadiene for adsorbing on the cathode surface. The electrostatic potential (ESP) diagram is obtained by molecular dynamics simulation, and the results point out that the potential distributes at 35 ~ 78 kcal·mol-1, meaning the easily adsorption of additive MG on the cathode surface. The complex of CoMG was formed from delocalized pi-bond of phenyl of MG and free Co ion in the bath.

/
| 〈 |
|
〉 |