

基于非亲核电解液构建稳定的镁离子电池
收稿日期: 2021-12-28
修回日期: 2022-01-20
网络出版日期: 2022-03-22
版权
An Additive Incorporated Non-Nucleophilic Electrolyte for Stable Magnesium Ion Batteries
# Xie M L and Wang J contributed equally to this work.
Received date: 2021-12-28
Revised date: 2022-01-20
Online published: 2022-03-22
Copyright
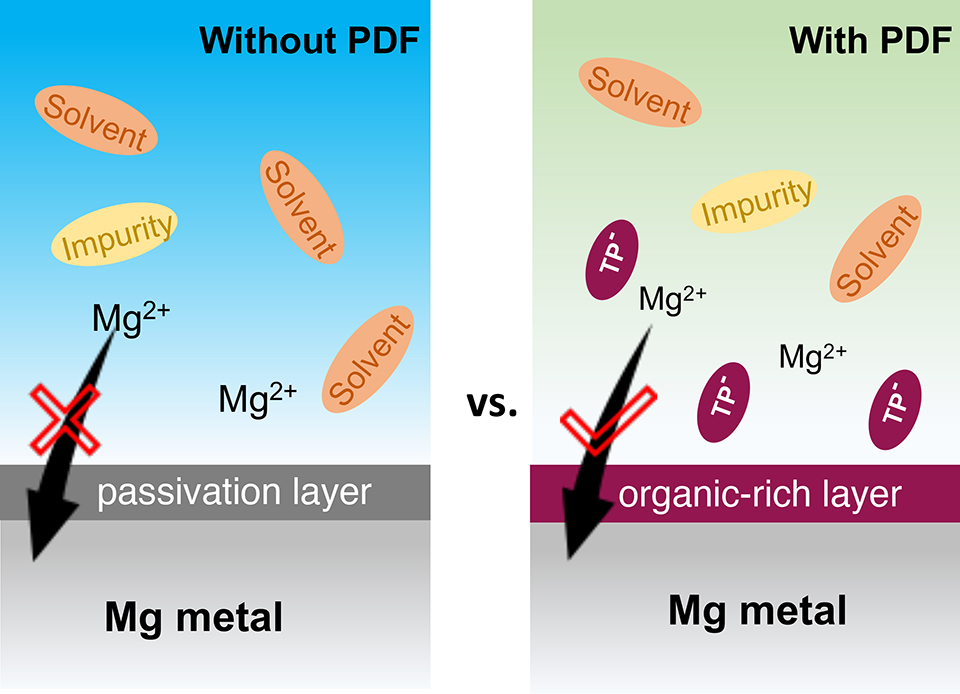
非亲核电解液被认为是新一代可用于镁离子电池的高稳定电解液。但由于电解液容易在镁金属表面产生不传导镁离子的钝化层,导致镁的电化学沉积/溶出过程在该电解液中表现出动力学缓慢、库仑效率较低等缺点。在本研究中,我们通过在非亲核电解液中引入二苯二硫醚(PDF)添加剂以实现对镁金属电极的界面调控。研究表明PDF产生的苯基硫醇盐中间体可以紧密结合在镁金属表面,并显著抑制了镁金属表面钝化层的生成。经界面优化后的镁金属电极的沉积-溶出库仑效率高达99.5%,并且表现出显著降低的过电位。利用此电解液,并以镁金属为负极、Mo6S7Se为正极构建的镁离子电池在室温下可稳定循环150周(0.1 C)。这类通过添加剂优化镁金属界面的策略也将有助于推进其他类型的镁离子电解液的实际应用。
谢茂玲 , 王钧 , 胡晨吉 , 郑磊 , 孔华彬 , 沈炎宾 , 陈宏伟 , 陈立桅 . 基于非亲核电解液构建稳定的镁离子电池[J]. 电化学, 2022 , 28(3) : 2108561 . DOI: 10.13208/j.electrochem.210856
Non-nucleophilic electrolytes are promising next-generation highly stable electrolytes for magnesium-ion batteries (MIBs). However, a passivation layer on Mg metal anode usually blocks Mg2+ diffusion, leading to poor reaction kinetics and low Coulombic efficiency of the Mg plating/stripping in these electrolytes. Here we explore the utilization of phenyl disulfide (PDF) as a film-forming additive for non-nucleophilic electrolytes to regulate the interfacial chemistry on Mg metal anode. Phenyl-thiolate generated from the PDF additive was found to suppress the unfavorable surface blocking layer, resulted in a high Coulombic efficiency of up to 99.5% for the Mg plating/stripping process as well as a remarkably decreased overpotential. The full battery consisting of Mg metal anode and Mo6S7Se cathode remained stable in the PDF additive-containing electrolyte at 0.1 C over 150 cycles at room temperature.

/
| 〈 |
|
〉 |