

原位57Fe穆斯堡尔光谱技术及其在Ni-Fe基析氧反应电催化剂中的应用
收稿日期: 2021-12-14
修回日期: 2022-01-24
网络出版日期: 2022-02-22
版权
In-Situ/Operando 57Fe Mössbauer Spectroscopic Technique and Its Applications in NiFe-based Electrocatalysts for Oxygen Evolution Reaction
Received date: 2021-12-14
Revised date: 2022-01-24
Online published: 2022-02-22
Copyright
近年来,析氧反应(oxygen evolution reaction)中针对高效且具有成本效益的电催化剂开发一直是构筑有效利用可再生能源存储系统和水分解生产清洁氢能燃料的重大障碍。OER过程涉及四电子、四质子耦合并形成氧-氧(O-O)键,因此动力学上进程缓慢。为提升其在水分解产氢及二氧化碳还原反应中的应用,需要开发高效催化剂,降低OER过电位,以减轻能量转换过程中固有的能量损失。研究表明,IrO2和RuO2具有较低析氧过电位,但储量低、价格昂贵,大大限制了其在析氧反应中的大规模应用。而Ni-Fe基析氧催化剂在碱性水分解反应中展现了优异的性能,其在水分解过程中的催化机制仍有待进一步研究。
为了解决Ni-Fe基催化剂在析氧反应过程中反应位点及催化反应机制等关键问题,迫切需要更先进的原位技术来准确表征,原位追踪催化剂形态变化与电解质/电极之间的界面相互作用的影响。光谱与电化学结合的原位技术可以监测析氧反应过程催化剂自身的变化。目前,已有大量原位光谱技术与电化学进行结合,揭示Ni-Fe基催化剂在OER过程中的反应机理及活性位点,包括原位表面增强拉曼光谱、原位同步辐射X射线吸收光谱、原位紫外-可见光谱、原位扫描电化学显微镜及原位穆斯堡尔光谱等。其中,原位拉曼技术可以观察Ni-Fe催化剂的振动,可以在电解液中施加测试电压条件下监测电化学反应过程中的中间体,从而提供实时反应信息,有助于追踪电化学驱动反应是如何发生的。原位同步辐射技术可以研究OER过程中Ni-Fe催化剂材料的电子结构和局部几何结构的信息,但目前的研究中更多的是探究Ni的价态变化,对Fe的研究信息较少。原位紫外-可见光谱也主要是针对Ni(OH)2的变化展开研究,逐渐提高施加电位,Ni(OH)2会向着NiOOH逐渐变化,紫外-可见技术可以追踪Ni-Fe基电催化剂中的金属氧化过程。众多电化学原位光谱技术中,57Fe穆斯堡尔谱因具有超高的能量分辨率,是确定催化剂相结构、鉴定活性位点、阐明催化机理以及确定催化活性与催化剂配位结构之间关系的最佳手段。此外,原位穆斯堡尔光谱技术基于原子核和核外电子的超精细相互作用而给出的同质异能移、四极矩分裂以及有效磁场等针对催化剂中的Fe位点的氧化态、电子自旋构型、对称性和磁性信息进行研究,为Ni-Fe基催化剂在析氧反应中的应用提供强有力的支持。
1957年,德国科学家鲁道夫·路德维希·穆斯堡尔(Rudolf Ludwig Mössbauer)在其27岁时,发现作为晶格谐振子的原子在发射或吸收γ射线时以一定的概率不会改变它们的量子力学状态,而这一γ射线的核共振吸收现象于1961年获得诺贝尔物理学奖,不久后被命名为穆斯堡尔效应。穆斯堡尔效应是来自于无反冲的γ射线吸收和发射的核共振现象,能量Ee处于激发态的原子核(Z质子和N中子)通过产生能量为Eγ的γ射线跃迁到能量为Eg的基态,γ射线可能会被处于基态的另一个相同类型的原子核(相同的Z和N)吸收,从而转变为能量Ee的激发态。只有当发射线和吸收线足够重叠时,才能看到共振吸收。
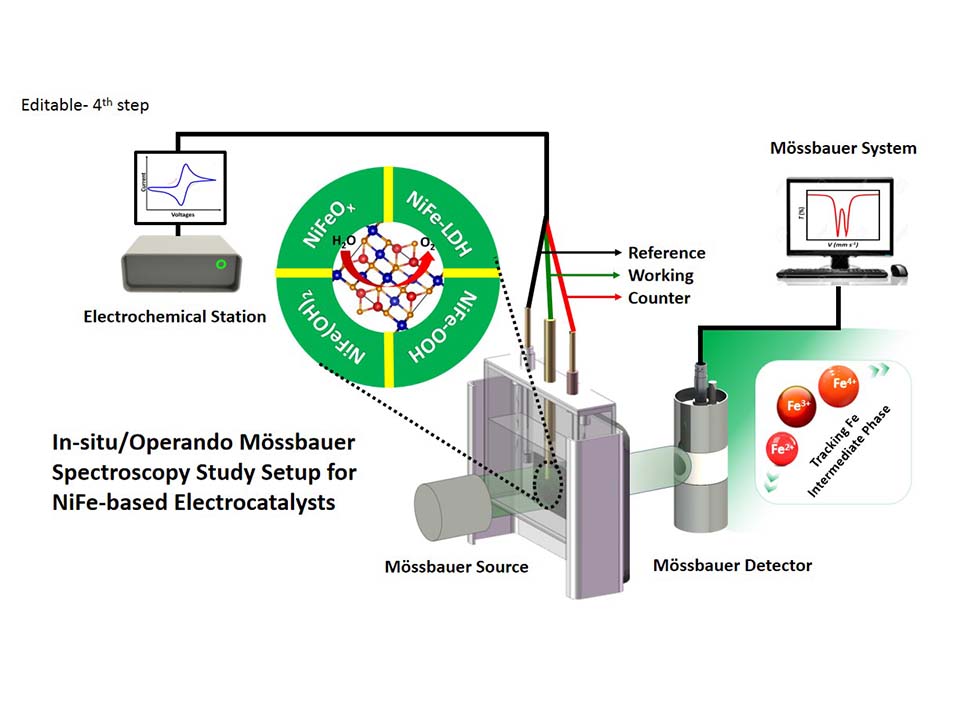
原位穆斯堡尔谱在Ni-Fe催化剂析氧反应中应用,首先需要搭建57Fe穆斯堡尔谱仪与电化学工作站联用。标准的穆斯堡尔光谱仪主要由放射源(通常是57Co在Rh或Pd金属基质中用于57Fe穆斯堡尔光谱)、速度传感器、速度校准装置、波形发生器和同步器、γ射线检测系统、多通道分析仪、计算机,并且可选配低温恒温器或高温烘箱,以控制测量过程处于适宜温度。实际测试过程中,穆斯堡尔谱可以通过速度扫描方法生成,利用移动驱动器或速度传感器以特定速度重复移动源或样品(所谓的多普勒运动),同时γ射线连续传输或发射穿过样品并计数在同步通道上。获得穆斯堡尔谱图后,基于穆斯堡尔谱数据库(https://medc.dicp.ac.cn/,由中国科学院大连化学研究所穆斯堡尔效应数据中心从全世界收集的穆斯堡尔谱样品数据),对57Fe穆斯堡尔谱进行分析拟合,对含Fe基材料的物相、价态、自旋态和配位结构进行归因和分析。数据分析拟合主要利用MossWinn数据分析和拟合软件(http://www.mosswinn.com/)。以Ni-Fe氢氧化物催化剂为例,对于原始催化剂,其仅存在一种Fe3+物种,当该催化剂参与OER过程后,可能会存在Fe4+,在双峰基础上,拟合结果中则会出现肩峰向负侧移动现象,可以确认高价Fe的存在,例如Fe4+。为充分证明高价Fe的存在,对于Ni-Fe基催化剂的穆斯堡尔谱测试,还需在工况条件下进行原位测试。
20世纪80年代后期,非贵金属氧化物和氢氧化物代替贵金属氧化物阳极催化剂的电解水研究开始受到关注。Corrigan等通过将Fe杂质引入NiO阳极,测试过程中发现OER活性会增加,但后续的研究中对于Fe究竟如何改变Ni基催化剂的OER性能仍旧不清晰。尔后,原位穆斯堡尔谱的引入逐渐揭开Fe在Ni-Fe电催化水分解析氧反应中的作用。为提高测试准确性并保证穆斯堡尔谱信号的稳定,本实验室对原位穆斯堡尔谱装置做了开发和改进。主要包括三部分:(1) 穆斯堡尔光谱仪,(2) 电化学工作站,以及(3) 自主设计的原位OER电化学反应池。在我们的实验室中,使用了具有14.4 keV级γ射线的单线57Fe穆斯堡尔谱放射源57Co(Rh),可以减少电解液中的信号衰减并获得令人满意的信噪比,附带CHI660E电化学工作站。对于常规的OER测试,在室温298 K条件下进行测试,测试前首先用α-Fe对穆斯堡尔谱仪进行多普勒速度校准,在进行原位穆斯堡尔谱-OER实验之前,电解液用氮气或氩气饱和以去除溶解的氧气。为了保证测试信号的准确性,实验中所使用的电解池不含任何Fe杂质,因此采用了Teflon材料。为避免测试过程中产生的O2气泡对信号产生干扰,可以采用蠕动泵循环电解液,并且保证测试过程中局部的微反应环境的一致性。对于普通OER测试,仅需要少量催化剂,但对于原位57Fe穆斯堡尔谱测试,只有保证Ni-Fe催化剂中57Fe含量充足的条件下,才可以获得高质量信号。但OER过程中,不建议催化剂载量过高,催化过程中主要是表面催化剂在反应,当样品过厚时,深层样品无法参与析氧反应过程,可能会有部分Fe仍旧维持Fe3+状态。通常,对于常规57Fe穆斯堡尔光谱测量的催化剂,若在制备中使用普通Fe源,则需要Fe含量在5 ~ 10 mg·cm-2,这其中仅有~2.2%的自然丰度57Fe同位素,需要长时间监测才可以采集到信号。为保证实验的顺利进行,可以在样品制备过程中直接使用57Fe源,方便快捷采集高质量信号。为了保证样品测试的准确性,在OER开始前,我们可以在同一电解液中,在开路电位(OCP)下,对其进行测试,这一原始样品的测试可与后续施加电位的Ni-Fe催化剂测试结果进行对比。有外加电压测试时,需要保证催化剂处于稳定状态下进行测试,整个测试过程中保持电流密度稳定,这不仅可以保证催化剂的稳定性,还有助于确定催化剂的真实结构。
利用原位57Fe穆斯堡尔谱,我们对通过Ni-Fe普鲁士蓝类似物原位拓扑转换获得的Ni-Fe羟基氧化物电催化剂进行了测试。基于原位拉曼技术,我们发现在阳极电位下,Ni-Fe催化剂中α-Ni(OH)2相会不可逆转变为γ-NiOOH。原位57Fe穆斯堡尔谱测试结果表明,在较低的施加电位(例如1.22 V 和1.32 V vs. RHE)下,Fe在NiFe0.2-OxHy中仅处于+3氧化态,其光谱结果与开路电位下NiFe0.2-OxHy谱图相似,其中只有一个双峰,两个峰的强度相等,可归因于高自旋 Fe3+物种。但随着外加电位增加并达到1.37 V,两个峰的强度开始变得不相等,开始出现一个小的肩峰,其同质异能移(δ)值约为-0.25 mm·s-1,可以归属为 Fe4+ 。随着电压的逐渐增加,催化剂中的Fe4+含量逐渐增加。在OER过程中,施加电位1.42 V vs. RHE时,Fe4+含量~ 12%。当施加的电势达到1.57 V时,催化剂中Fe4+的含量进一步增加到约40%。这一实例充分展现了原位57Fe穆斯堡尔谱与Ni-Fe催化OER过程的应用,也体现了NiFe0.2-OxHy催化剂原位产生的Fe4+物种的量与其水氧化反应性能呈正相关,进一步加深了对Ni-Fe水氧化催化机理的理解。
Ni-Fe基催化剂因其价格低廉,电催化析氧性能优异,因此成为碱性水分解析氧过程的理想候选者。虽然Ni-Fe基电催化剂表现出优异的OER活性,但缺乏长期稳定性阻碍了其在商业中的应用。因此,充分了解Ni-Fe催化剂的衰减机理,包括形态、组成、晶体结构和活性位点数量的变化,对于设计稳定和高效Ni-Fe催化材料非常重要,充分了解Ni-Fe催化剂在OER过程中的电子结构及其与析氧反应中间体的相互作用尤为重要。原位拉曼及原位紫外-可见光谱可以对Ni-Fe催化剂中的Ni(OH)2到NiOOH的变化进行深入探究,而原位57Fe穆斯堡尔谱测试则可以揭示Ni-Fe基催化剂中Fe的电子环境及其电子的、结构的和磁性的变化。穆斯堡尔光谱为研究Ni-Fe催化剂中Fe的局部电子结构、局部配位、键合和氧化态的提供了强大技术支撑。最近,穆斯堡尔光谱在电催化领域获得了越来越多的关注,它对于检测不同铁基催化材料中的主要活性位点有着重要作用。
关键词: 析氧反应; Ni-Fe羟基氧化物电催化剂; 原位电化学穆斯堡尔光谱技术; Ni-Fe普鲁士蓝类似物; 关键中间物表征
Jafar Hussain Shah , 谢起贤 , 匡智崇 , 格日乐 , 周雯慧 , 刘朵绒 , Alexandre I. Rykov , 李旭宁 , 罗景山 , 王军虎 . 原位57Fe穆斯堡尔光谱技术及其在Ni-Fe基析氧反应电催化剂中的应用[J]. 电化学, 2022 , 28(3) : 2108541 . DOI: 10.13208/j.electrochem.210854
The development of highly efficient and cost-effective electrocatalysts for the sluggish oxygen evolution reaction (OER) remains a significant barrier to establish effective utilization of renewable energy storage systems and water splitting to produce clean fuel. The current status of the research in developing OER catalysts shows that NiFe-based oxygen evolution catalysts (OECs) have been proven as excellent and remarkable candidates for this purpose. But it is critically important to understand the factors that influence their activity and underlying mechanism for the development of state-of-the-art OER catalysts. Therefore, the development of in-situ/operando characterizations is urgently required to detect key intermediates along with active sites and phases responsible for OER. 57Fe Mössbauer spectroscopy is one of the appropriate and suitable techniques for determining the phase structure of catalysts under their electrochemical working conditions, identifying the active sites, clarifying the catalytic mechanisms, and determining the relationship between catalytic activity and the coordination structure of catalysts. In this tutorial review, we have discussed the current status of research on NiFe-based catalysts with particular attention to introduce in detail the knowhow about the development and utilization of in-situ/operando57Fe Mössbauer-electrochemical spectroscopy for the study of OER mechanism. A brief overview using NiFe-(oxy)hydroxide catalysts, derived from ordered porous metal-organic framework (MOF) material NiFe-PBAs (Prussian blue analogues), as a typical model study case for the OER electrocatalyst and self-designed in-situ/operando57Fe Mössbauer-electrochemical instrument, has been provided for the better understanding of readers. Moreover, using in-situ/operando57Fe Mössbauer spectroscopy, the crucial role of Fe species during OER reaction has been explained very well.

| [1] | Chu S, Majumdar A. Opportunities and challenges for a sustainable energy future[J]. Nature, 2012, 488(7411):294-303. |
| [2] | Fu J, Cano Z P, Park M G, Yu A, Fowler M, Chen Z W. Electrically rechargeable zinc-air batteries: progress, challenges, and perspectives[J]. Adv. Mater., 2017, 29(7):1604685. |
| [3] | Zhang H W, Shen P K. Recent development of polymer electrolyte membranes for fuel cells[J]. Chem. Rev., 2012, 112(5):2780-2832. |
| [4] | Jiao K, Xuan J, Du Q, Bao Z M, Xie B A, Wang B W, Zhao Y, Fan L H, Wang H Z, Hou Z L, Huo S, Brandon N P, Yin Y, Guiver M D. Designing the next generation of proton-exchange membrane fuel cells[J]. Nature, 2021, 595(7867):361-369. |
| [5] | Johnson D, Qiao Z, Djire A. Progress and challenges of carbon dioxide reduction reaction on transition metal based electrocatalysts[J]. ACS Appl. Energy Mater., 2021, 4(9):8661-8684. |
| [6] | Li Y J, Sun Y J, Qin Y N, Zhang W Y, Wang L, Luo M C, Yang H, Guo S J. Recent advances on water-splitting electrocatalysis mediated by noble-metal-based nanostructured materials[J]. Adv. Energy Mater., 2020, 10(11):1903120. |
| [7] | Li J C, Kuang Y, Meng Y T, Tian X, Hung W H, Zhang X, Li A W, Xu M Q, Zhou W, Ku C S, Chiang C Y, Zhu G Z, Guo J Y, Sun X M, Dai H J. Electroreduction of CO2 to formate on a copper-based electrocatalyst at high pressures with high energy conversion efficiency[J]. J. Am. Chem. Soc., 2020, 142(16):7276-7282. |
| [8] | Wang M Y, Wang Z, Gong X Z, Guo Z C. The intensification technologies to water electrolysis for hydrogen production-A review[J]. Renew. Sust. Energ. Rev., 2014, 29:573-588. |
| [9] | Mei L, Gao X P, Gao Z, Zhang Q Y, Yu X G, Rogach A L, Zeng Z Y. Size-selective synjournal of platinum nanoparticles on transition-metal dichalcogenides for the hydrogen evolution reaction[J]. Chem. Commun., 2021, 57(23):2879-2882. |
| [10] | Yu J, He Q J, Yang G M, Zhou W, Shao Z P, Ni M. Recent advances and prospective in ruthenium-based materials for electrochemical water splitting[J]. ACS Catal., 2019, 9(11):9973-10011. |
| [11] | Hu C L, Zhang L, Gong J L. Recent progress made in the mechanism comprehension and design of electrocatalysts for alkaline water splitting[J]. Energy Environ. Sci., 2019, 12(9):2620-2645. |
| [12] | Lyons M E G, Floquet S. Mechanism of oxygen reactions at porous oxide electrodes. Part 2-Oxygen evolution at RuO2, IrO2 and IrxRu1-xO2 electrodes in aqueous acid and alkaline solution[J]. Phys. Chem. Chem. Phys., 2011, 13(12):5314-5335. |
| [13] | Hunter B M, Gray H B, Muller A M. Earth-abundant heterogeneous water oxidation catalysts[J]. Chem. Rev., 2016, 116(22):14120-14136. |
| [14] | Suen N T, Hung S F, Quan Q, Zhang N, Xu Y J, Chen H M. Electrocatalysis for the oxygen evolution reaction: recent development and future perspectives[J]. Chem. Soc. Rev., 2017, 46(2):337-365. |
| [15] | Lee Y, Suntivich J, May K J, Perry E E, Shao-Horn Y. Synjournal and activities of rutile IrO2 and RuO2 nanoparticles for oxygen evolution in acid and alkaline solutions[J]. J. Phys. Chem. Lett., 2012, 3(3):399-404. |
| [16] | Blakemore J D, Schley N D, Kushner-Lenhoff M N, Winter A M, D’Souza F, Crabtree R H, Brudvig G W. Comparison of amorphous iridium water-oxidation electrocatalysts prepared from soluble precursors[J]. Inorg. Chem., 2012, 51(14):7749-7763. |
| [17] | Dionigi F, Zhu J, Zeng Z H, Merzdorf T, Sarodnik H, Gliech M, Pan L J, Li W X, Greeley J, Strasser P. Intrinsic electrocatalytic activity for oxygen evolution of crystalline 3d-transition metal layered double hydroxides[J]. Angew. Chem. Int. Edit., 2021, 60(26):14446-14457. |
| [18] | Han L, Dong S L, Wang E K. Transition-metal (Co, Ni, and Fe)-based electrocatalysts for the water oxidation reaction[J]. Adv. Mater., 2016, 28(42):9266-9291. |
| [19] | Zhang Q Y, Mei L, Cao X H, Tang Y X, Zeng Z Y. Intercalation and exfoliation chemistries of transition metal di-chalcogenides[J]. J. Mater. Chem. A, 2020, 8(31):15417-15444. |
| [20] | Han N, Liu P Y, Jiang J, Ai L H, Shao Z P, Liu S M. Recent advances in nanostructured metal nitrides for water splitting[J]. J. Mater. Chem. A, 2018, 6(41):19912-19933. |
| [21] | Zhang H J, Maijenburg A W, Li X P, Schweizer S L, Wehrspohn R B. Bifunctional heterostructured transition metal phosphides for efficient electrochemical water splitting[J]. Adv. Funct. Mater., 2020, 30(34):2003261. |
| [22] | Gupta S, Patel M K, Miotello A, Patel N. Metal boride-based catalysts for electrochemical water-splitting: A review[J]. Adv. Funct. Mater., 2020, 30(1):1906481. |
| [23] | Wang H P, Zhu S, Deng J W, Zhang W C, Feng Y Z, Ma J M. Transition metal carbides in electrocatalytic oxygen evolution reaction[J]. Chin. Chem. Lett., 2021, 32(1):291-298. |
| [24] | Zhang B, Zheng Y J, Ma T, Yang C D, Peng Y F, Zhou Z H, Zhou M, Li S, Wang Y H, Cheng C. Designing MOF nanoarchitectures for electrochemical water splitting[J]. Adv. Mater., 2021, 33(17):2006042. |
| [25] | Balogun M S, Huang Y C, Qiu W T, Yang H, Ji H B, Tong Y X. Updates on the development of nanostructured transition metal nitrides for electrochemical energy storage and water splitting[J]. Mater. Today, 2017, 20(8):425-451. |
| [26] | Jin S. Are metal chalcogenides, nitrides, and phosphides oxygen evolution catalysts or bifunctional catalysts?[J] ACS Energy Lett., 2017, 2(8):1937-1938. |
| [27] | Dionigi F, Strasser, P. NiFe-based (oxy)hydroxide catalysts for oxygen evolution reaction in non-acidic electrolytes[J]. Adv. Energy Mater., 2016, 6(23):1600621. |
| [28] | Gong M, Li Y G, Wang H L, Liang Y Y, Wu J Z, Zhou J G, Wang J, Regier T, Wei F, Dai H J. An advanced Ni-Fe layered double hydroxide electrocatalyst for water oxidation[J]. J. Am. Chem. Soc., 2013, 135(23):8452-8455. |
| [29] | Subbaraman R, Tripkovic D, Chang K C, Strmcnik D, Paulikas A P, Hirunsit P, Chan M, Greeley J, Stamenkovic V, Markovic N M. Trends in activity for the water electrolyser reactions on 3d M (Ni, Co, Fe, Mn) hydr(oxy)oxide catalysts[J]. Nat. Mater., 2012, 11(6):550-557. |
| [30] | Munshi M Z A, Tseung A C C, Parker J. The dissolution of iron from the negative material in pocket plate nickel-cadmium batteries[J]. J. Appl. Electrochem., 1985, 15(5):711-717. |
| [31] | Tichenor R L. Nickel oxides-relation between electrochemical and foreign ion content[J]. J. Ind. Eng. Chem., 1952, 44(5), 973-977. |
| [32] | Corrigan D A. The catalysis of the oxygen evolution reaction by iron impurities in thin film nickel oxide electrodes[J]. J. Electrochem. Soc., 1987, 134(2):377-384. |
| [33] | Zhu K Y, Liu H Y, Li M R, Li X N, Wang J H, Zhu X F, Yang W S. Atomic-scale topochemical preparation of crystalline Fe3+-doped β-Ni(OH)2 for an ultrahigh-rate oxygen evolution reaction[J]. J. Mater. Chem. A, 2017, 5(17):7753-7758. |
| [34] | Stevens M B, Trang C D M, Enman L J, Deng J, Boettcher S W. Reactive Fe-sites in Ni/Fe(oxy)hydroxide are respon-sible for exceptional oxygen electrocatalysis activity[J]. J. Am. Chem. Soc., 2017, 139(33):11361-11364. |
| [35] | Młynarek G, Paszkiewicz M, Radniecka A. The effect of ferric ions on the behaviour of a nickelous hydroxide electrode[J]. J. Appl. Electrochem., 1984, 14(2):145-149. |
| [36] | Trotochaud L, Young S L, Ranney J K, Boettcher S W. Nickel-iron oxyhydroxide oxygen-evolution electrocatalysts: the role of intentional and incidental iron incorporation[J]. J. Am. Chem. Soc., 2014, 136(18):6744-6753. |
| [37] | Görlin M, Chernev P, de Araújo J F, Reier T, Dresp S, Paul B, Krähnert R, Dau H, Strasser P. Oxygen evolution reaction dynamics, faradaic charge efficiency, and the active metal redox states of Ni-Fe oxide water splitting electrocatalysts[J]. J. Am. Chem. Soc., 2016, 138(17):5603-5614. |
| [38] | Görlin M, de Araújo J F, Schmies H, Bernsmeier D, Dresp S, Gliech M, Jusys Z, Chernev P, Kraehnert R, Dau H, Strasser P. Tracking catalyst redox states and reaction dynamics in Ni-Fe oxyhydroxide oxygen evolution reaction electrocatalysts: the role of catalyst support and electrolyte pH[J]. J. Am. Chem. Soc., 2017, 139(5):2070-2082. |
| [39] | Hunter B M, Thompson N B, Müller A M, Rossman G R, Hill M G, Winkler J R, Gray H B. Trapping an iron(VI) water-splitting intermediate in nonaqueous media[J]. Joule, 2018, 2(4):747-763. |
| [40] | Ahn H S, Bard A J. Surface interrogation scanning electrochemical microscopy of Ni1-xFexOOH (0 < x < 0.27) ox-ygen evolving catalyst: Kinetics of the “fast” iron sites[J]. J. Am. Chem. Soc., 2016, 138(1):313-318. |
| [41] | Klaus S, Cai Y, Louie M W, Trotochaud L, Bell A T. Effects of Fe electrolyte impurities on Ni(OH)2/NiOOH structure and oxygen evolution activity[J]. J. Phys. Chem. C, 2015, 119(13):7243-7254. |
| [42] | Zou S H, Burke M S, Kast M G, Fan J, Danilovic N, Bo-ettcher S W. Fe (oxy) hydroxide oxygen evolution reaction electrocatalysis: intrinsic activity and the roles of electrical conductivity, substrate, and dissolution[J]. Chem. Mater., 2015, 27(23):8011-8020. |
| [43] | Friebel D, Louie M W, Bajdich M, Sanwald K E, Cai Y, Wise A M, Cheng M J, Sokaras D, Weng T C, Alonso-Mori R, Davis R C, Bargar J R, Norskov J K, Nilsson A, Bell A T. Identification of highly active Fe sites in (Ni, Fe) OOH for electrocatalytic water splitting[J]. J. Am. Chem. Soc., 2015, 137(3):1305-1313. |
| [44] | Chen S C, Kang Z X, Zhang X D, Xie J F, Wang H, Shao W, Zheng X S, Yan W S, Pan B C, Xie Y. Highly active Fe sites in ultrathin pyrrhotite Fe7S8 nanosheets realizing efficient electrocatalytic oxygen evolution[J]. ACS Central Sci., 2017, 3(11):1221-1227. |
| [45] | Xiao H, Shin H, Goddard W A. Synergy between Fe and Ni in the optimal performance of (Ni,Fe)OOH catalysts for the oxygen evolution reaction[J]. Proc. Natl. Acad. Sci. U.S.A., 2018, 115(23):5872-5877. |
| [46] | Corrigan D A, Conell R S, Fierro C A, Scherson D A. In-situ Moessbauer study of redox processes in a composite hydroxide of iron and nickel[J]. J. Phys. Chem., 1987, 91(19):5009-5011. |
| [47] | Chen J Y C, Dang L N, Liang H F, Bi W L, Gerken J B, Jin S, Alp E E, Stahl S S. Operando analysis of NiFe and Fe oxyhydroxide electrocatalysts for water oxidation: Detection of Fe4+ by Mössbauer spectroscopy[J]. J. Am. Chem. Soc., 2015, 137(48):15090-15093. |
| [48] | Tao H B, Xu Y H, Huang X, Chen J Z, Pei L J, Zhang J M, Chen J G G, Liu B. A general method to probe oxygen evolution intermediates at operating conditions[J]. Joule, 2019, 3(6):1498-1509. |
| [49] | Su X Z, Wang Y, Zhou J, Gu S Q, Li J, Zhang S. Operando spectroscopic identification of active sites in NiFe prussian blue analogues as electrocatalysts: Activation of oxygen atoms for oxygen evolution reaction[J]. J. Am. Chem. Soc., 2018, 140(36):11286-11292. |
| [50] | Shinagawa T, Garcia-Esparza A T, Takanabe K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion[J]. Sci. Rep., 2015, 5:13801. |
| [51] | Deng Y L, Yeo B S. Characterization of electrocatalytic water splitting and CO2 reduction reactions using in situ/operando Raman spectroscopy[J]. ACS Catal., 2017, 7(11):7873-7889. |
| [52] | Timoshenko J, Cuenya B R. In situ/operando electrocatalyst characterization by X-ray absorption spectroscopy[J]. Chem. Rev., 2020, 121(2):882-961. |
| [53] | Li X N, Wang H Y, Yang H B, Cai W Z, Liu S, Liu B. In situ/operando characterization techniques to probe the electrochemical reactions for energy conversion[J]. Small Methods, 2018, 2(6):1700395. |
| [54] | Zeng Y Q, Li X N, Wang J H, Sougrati M T, Huang Y Q, Zhang T, Liu B. In situ/operando Mössbauer spectroscopy for probing heterogeneous catalysis[J]. Chem. Catal., 2021, 1(6):1215-1233. |
| [55] | Qiu Z, Tai C W, Niklasson G A, Edvinsson T. Direct observation of active catalyst surface phases and the effect of dynamic self-optimization in NiFe-layered double hydroxides for alkaline water splitting[J]. Energy Environ. Sci., 2019, 12(2):572-581. |
| [56] | Stoerzinger K A, Hong W T, Crumlin E J, Bluhm H, Shao-Horn Y. Insights into electrochemical reactions from ambient pressure photoelectron spectroscopy[J]. Accounts Chem. Res., 2015, 48(11):2976-2983. |
| [57] | Ali-Löytty H, Louie M W, Singh M R, Li L, Casalongue H G S, Ogasawara H, Crumlin E J, Liu Z, Bell A T, Nilsson A, Friebel D. Ambient-pressure XPS study of a Ni-Fe electrocatalyst for the oxygen evolution reaction[J]. J. Phys. Chem. C, 2016, 120(4):2247-2253. |
| [58] | Li X N, Zhu K Y, Pang J F, Tian M, Liu J Y, Rykov A I, Zheng M Y, Wang X D, Zhu X F, Huang Y Q, Liu B, Wang J H, Yang W S, Zhang T. Unique role of Mössbauer spectroscopy in assessing structural features of heterogeneous catalysts[J]. Appl. Catal. B-Environ., 2018, 224:518-532. |
| [59] | Mössbauer R L. Kernresonanzfluoreszenz von gammastr-ahlung in Ir191[J]. Zeitschrift für Physik, 1958, 151(2):124-143. |
| [60] | Liu K, Rykov A I, Wang J H, Zhang T. Recent advances in the application of Mössbauer spectroscopy in heterogeneous catalysis[J]. Adv. Catal., 2015, 58:1-142. |
| [61] | Kramm U I, Ni L M, Wagner S. 57Fe Mössbauer spectroscopy characterization of electrocatalysts[J]. Adv. Ma-ter., 2019, 31(31):1805623. |
| [62] | Fischer N, Claeys M. In situ characterization of Fischer-Tropsch catalysts: A review[J]. J. Phys. D-Appl. Phys., 2020, 53(29):293001. |
| [63] | Gütlich P, Bill E, Trautwein A X. Mössbauer spectroscopy and transition metal chemistry: Fundamentals and applications[M]. Deutschland: Springer, 2010. |
| [64] | Wang J H, Jin C Z, Liu X, Liu D R, Sun H, Wei F F, Zhang T, Stevens J G, Khasanov A, Khasanova I. Mössbauer spectroscopy database: Past, present, future[J]. Hyperfine Interact., 2012, 204(1-3):111-117. |
| [65] | Klencsár Z. MossWinn-methodological advances in the field of Mössbauer data analysis[J]. Hyperfine Interact., 2013, 217(1-3):117-126. |
| [66] | Kuang Z C, Liu S, Li X N, Wang M, Ren X Y, Ding J, Ge R L, Zhou W H, Rykov A I, Sougrati M T, Lippens P E, Huang Y Q, Wang J H. Topotactically constructed nickel-iron (oxy)hydroxide with abundant in-situ produced high-valent iron species for efficient water oxidation[J]. J. Energy Chem., 2021, 57:212-218. |
| [67] | Li X N, Ao Z M, Liu J Y, Sun H Q, Rykov A I, Wang J H. Topotactic transformation of metal-organic frameworks to graphene-encapsulated transition-metal nitrides as efficient Fenton-like catalysts[J]. ACS Nano, 2016, 10(12):11532-11540. |
| [68] | Catala L, Mallah T. Nanoparticles of Prussian blue analogs and related coordination polymers: From information storage to biomedical applications[J]. Coord. Chem. Rev., 2017, 346:32-61. |
| [69] | Zakaria M B, Chikyow T. Recent advances in Prussian blue and Prussian blue analogues: Synjournal and thermal treatments[J]. Coord. Chem. Rev., 2017, 352:328-345. |
| [70] | Hu M, Belik A A, Imura M, Mibu K, Tsujimoto Y, Yamauchi Y. Synjournal of superparamagnetic nanoporous iron oxide particles with hollow interiors by using prussian blue coordination polymers[J]. Chem. Mater., 2012, 24(14):2698-2707. |
| [71] | Li X N, Wang Z H, Zhang B, Rykov A I, Ahmed M A, Wang J H. FexCo3-xO4 nanocages derived from nanoscale metal-organic frameworks for removal of bisphenol A by activation of peroxymonosulfate[J]. Appl. Catal. B-Environ., 2016, 181:788-799. |
| [72] | Li X N, Cao C S, Hung S F, Lu Y R, Cai W Z, Rykov A I, Miao S, Xi S B, Yang H B, Hu Z H, Wang J H, Zhao J Y, Alp E E, Xu W, Chan T S, Chen H M, Xiong Q H, Xiao H, Huang Y Q, Li J, Zhang T, Liu B. Identification of the electronic and structural dynamics of catalytic centers in single-Fe-atom material[J]. Chem, 2020, 6(12):3440-3454. |
| [73] | Li X N, Zeng Y Q, Tung C W, Lu Y R, Baskaran S, Hung S F, Wang S F, Xu C Q, Wang J H, Chan T S, Chen H M, Jiang J C, Yu Q, Huang Y Q, Li J, Zhang T, Liu B. Unveiling the in situ generation of a monovalent Fe(I) site in the single-Fe-atom catalyst for electrochemical CO2 reduction[J]. ACS Catal., 2021, 11(12):7292-7301. |
| [74] | Li J K, Sougrati M T, Zitolo A, Ablett J M, Oĝuz I C, Mineva T, Matanovic I, Atanassov P, Huang Y, Zenyuk I, Di Cicco A, Kumar K, Dubau L, Maillard F, Dražic G, Jaouen F. Identification of durable and non-durable FeNx sites in Fe-N-C materials for proton exchange membrane fuel cells[J]. Nature. Catal., 2020, 4(1):10-19. |
| [75] | Zhu K Y, Zhu X F, Yang W S. Application of in-situ techniques for the characterization of NiFe-based oxygen evolution reaction (OER) electrocatalysts[J]. Angew. Chem. Int. Ed., 2019, 58(5):1252-1265. |
| [76] | Gao M R, Sheng W C, Zhuang Z B, Fang Q R, Gu S, Jiang J, Yan Y S. Efficient water oxidation using nanostructured α-nickel-hydroxide as an electrocatalyst[J]. J. Am. Chem. Soc., 2014, 136(19):7077-7084. |
/
| 〈 |
|
〉 |