

铂片电极表面芥子气伏安行为研究
收稿日期: 2021-05-07
修回日期: 2021-05-22
网络出版日期: 2022-02-21
基金资助
国家自然科学基金(51551204);国家重点研发计划(2018YFB0104404)
Electrochemical Voltammetric Behavior of Sulfur Mustard on the Bare Pt Electrode
Received date: 2021-05-07
Revised date: 2021-05-22
Online published: 2022-02-21
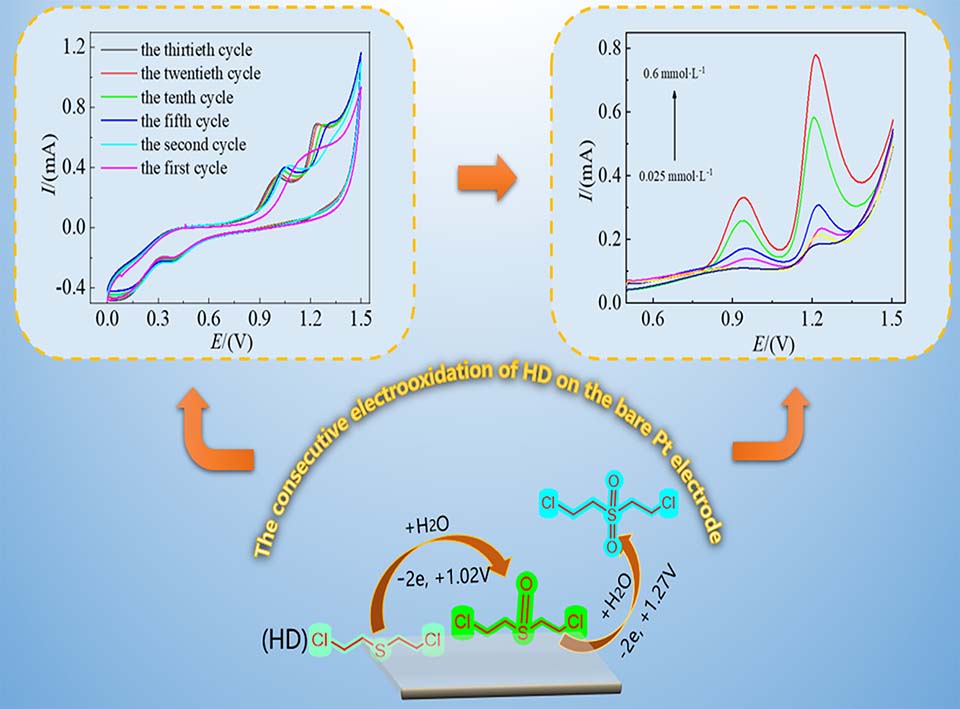
本文探究了芥子气(HD)在铂片电极表面的电化学行为。循环伏安(CV)结果展现了两个分隔良好的氧化峰,不同扫描速率的CV曲线表明氧化峰电流和峰电势均与扫描速率呈一定的线性关系,证明了该氧化过程为动力学控制的不可逆过程,同时计算了氧化过程转移的电子数和相关参数。以HD在铂片上的电化学行为为基础,采用傅里叶红外光谱(FT-IR)和电化学对比实验解释了CV曲线中两个氧化峰相对应的氧化过程, HD连续电化学氧化为二氯二乙基亚砜和二氯二乙基砜,为鉴别两种氧化产物提供了新的策略。方波伏安法(SWV)结果表明:HD浓度为2.5 × 10-5 ~ 6.0 × 10-4 mol·L-1时与峰电流呈线性关系,检测限为2.15 × 10-5 mol·L-1,该方法无需电极材料修饰,简单易得,且稳定性好,抗干扰能力强。通过研究HD在铂片电极表面的电化学行为,为其电化学传感提供基础。下一步工作应以负载型铂纳米粒子和多维载体为出发点,提高其检测能力。
杨育霖 , 孙杰 , 周添 , 李吉刚 , 卫寿平 . 铂片电极表面芥子气伏安行为研究[J]. 电化学, 2022 , 28(5) : 2105071 . DOI: 10.13208/j.electrochem.210507
The sulfur mustard (bis(2-chloroethyl) sulphide, HD), one of highly toxic chemical weapon agents, can damage the alive tissue cells (such as skin, lung, respiratory mucosa and so on), and cause carcinogenic and mutagenic effects for a long time exposure, which imposes a great threat not only to the human health, but also to the sustainable development of the society. With its convenience, high sensitivity and rapid response, electrochemical technology exhibits considerable potential in the field-deployed detection toward HD, but the related reports are rare. Herein, the electrochemical behavior of HD on the bare Pt electrode was investigated by electrochemical measurements, and cyclic voltammetric (CV) results exhibited two well-defined oxidation peaks. According to the CV curves at different scan rates, the calculated the amount of transfer electron (n) and transfer coefficient value (α) reveal that the oxidation of HD followed absorption-controlled kinetics. To investigate the electrochemical behavior of HD on the bare Pt electrode, FT-IR and comparative experiments were carried out. The HD oxidation processes corresponding to the two oxidation peaks in CV plots were explained. The results show that the oxidation peaks at 1.02 V and 1.27 V attributed to the oxidation formations of bis(2-chloroethyl) sulfoxide and bis(2-chloroethyl) sulfone, respectively. The difference of HD oxidation peaks potential provides a new strategy to identify bis(2-chloroethyl) sulfoxide and bis(2-chloroethyl) sulfone. Square wave voltammetry (SWV) was used to quantitatively analyze HD with bare Pt electrode as the working electrode, a linear dependence of anodic oxidation peak current versus HD concentration was obtained in the range of 2.5 × 10-5 ~ 6.0 × 10-4 mol·L-1, with a detection limit of 2.15 × 10-5 mol·L-1. Bare Pt, as the working electrode, which may not be modified furtherly, may distinguish the two oxidation peaks well. Additionally, the bare Pt working electrode demonstrated excellent anti-interfering ability in the presences of various inorganic ions and C6H12O6, as well as excellent stability. The investigation in the electrochemical behavior of HD will provide a foundation for the electrochemical sensor and degradation toward HD. The next work should focus on the improvements of linear range and limit detection with loaded Pt nanoparticle and multidimensional supports.

/
| 〈 |
|
〉 |