

碳限域Li3VO4纳米材料的制备及其储锂性能
收稿日期: 2021-12-02
修回日期: 2022-01-11
录用日期: 2022-01-12
网络出版日期: 2022-01-21
基金资助
国家自然科学基金项目(21805012);北京市自然科学基金项目(2182015);北京市大学生研究训练计划项目(2021J00129)
Preparation and Lithium Storage Properties of Carbon Confined Li3VO4 Nano Materials
Received date: 2021-12-02
Revised date: 2022-01-11
Accepted date: 2022-01-12
Online published: 2022-01-21
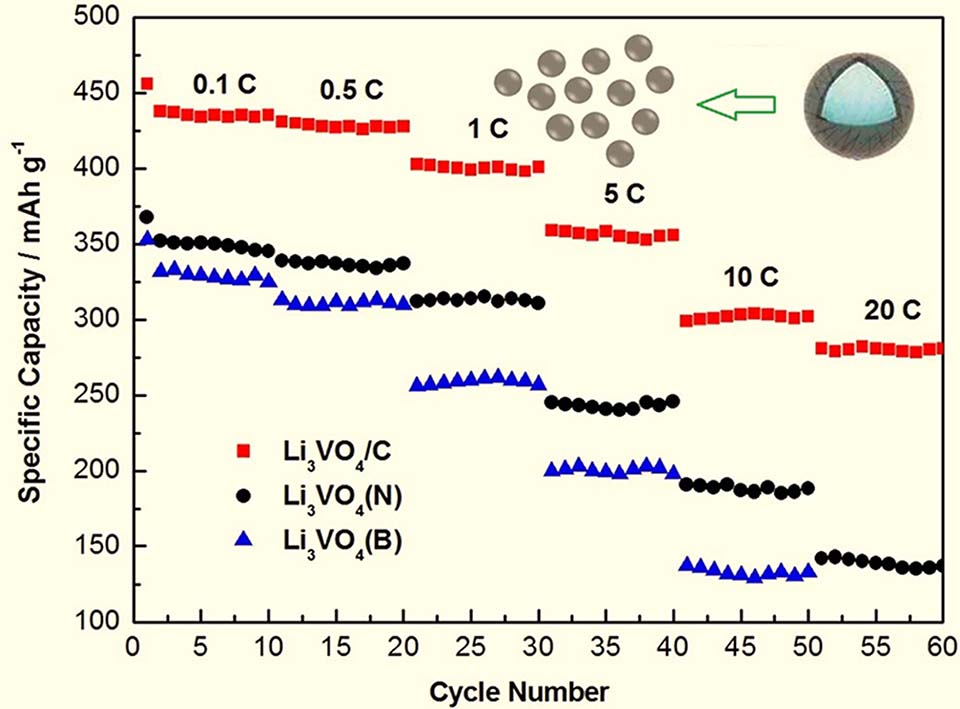
采用水热-固相两步合成法合成了碳限域Li3VO4纳米材料 (Li3VO4/C),并与相同的方法合成的非限域Li3VO4(N)纳米材料和固相法合成的Li3VO4(B)材料进行了对比。通过XRD,Raman,TEM,BET等表征方法,研究了所合成材料的组成、结构、形貌及比表面积,发现碳限域Li3VO4具有更小的晶粒大小,限域层的厚度为2-4 nm,且限域后提高了Li3VO4的比表面积和孔体积。用作锂离子电池负极材料时Li3VO4/C具有比Li3VO4(N)和Li3VO4(B)更高的储锂容量,更好的倍率性能和更稳定的循环性能。经分析认为,在Li3VO4/C材料中碳限域层减小了其在充放电过程中的欧姆极化,增大了材料的比表面积,因而提高了电解液的渗透效率和与活性材料的接触面积,缩短了锂离子的扩散路径,故提高了电化学性能。
范佳琪 , 宋焕巧 , 安佳莹 , 阿依达娜·阿曼太 , 陈默 . 碳限域Li3VO4纳米材料的制备及其储锂性能[J]. 电化学, 2023 , 29(11) : 211203 . DOI: 10.13208/j.electrochem.211203
Li3VO4, as a promising anode material for lithium ion batteries, has been widely studied because of its low and safe voltage, and large capacity. However, its poor electronic conductivity impedes the practical application of Li3VO4 particularly at high rates. In this paper, carbon confined Li3VO4 nano materials (Li3VO4/C) were synthesized by hydrothermal and solid-phase method, and for comparison, the Li3VO4 (N) nano materials without carbon confinement and Li3VO4 (B) materials were also synthesized by pure solid-phase method. The composition, structure, morphology and specific surface area of the three samples were studied by XRD, Raman, TEM and N2 adsorption-desorption tests. It was found that the grain size of Li3VO4 in Li3VO4/C was the smallest, which is 51 nm, the grain size of Li3VO4 in Li3VO4 (N) was the second (93 nm), and the grain size of Li3VO4 prepared by pure solid-phase method was the largest (113 nm). The thickness of carbon confinement layer in Li3VO4/C was 2-4 nm, which was uniformly coated on the surface of Li3VO4. And the specific surface area and pore size distribution of the three samples were measured by BET and BJH methods. It was found that the samples prepared by hydrothermal and solid-phase method had mesoporous structure, and the Li3VO4 prepared by a simple solid-phase method had the least porous structure. The BET specific surface area and the pore volume of the carbon confinement sample were larger than those of the sample without carbon confinement layer (30.49 m2·g-1 vs. 26.42 m2·g-1 and 0.12 cm3·g-1 vs. 0.05 cm3·g-1), which is in agreement with the smaller grains of Li3VO4/C by XRD analysis, indicating that the carbon layer limits the growth of Li3VO4 grains, so as to increase the contact area between active material and electrolyte when the sample is used as the anode material of lithium ion battery. The charge-discharge performances of the synthesized samples as anodes of lithium ion battery were studied. It was found that the Li3VO4/C electrode displayed faster lithium ion storage performance than Li3VO4 (N) and Li3VO4 (B) electrodes. At the rates of 0.1 C, 0.5 C, 1 C, 5 C, 10 C and 20 C, the discharge capacities of Li3VO4/C were 435, 428, 401, 356, 302 and 280 mAh·g-1, respectively. In particular, after 50 cycles at 5 C, Li3VO4/C still maintained 92.3% of the initial capacity, which fully reflects the characteristics of larger capacity, higher rate capability and better stability of Li3VO4/C electrode. By analyzing the relationship between morphology and electrochemical properties, it is considered that the carbon confinement layer reduces the ohmic polarization of Li3VO4 in the processes of charge and discharge, the large specific surface area improves the penetration efficiency of electrolyte, and the small particle size shortens the diffusion path of lithium ions. At the same time, the synthesis method in this work presents a universal strategy for the preparation of other transition metal oxide salts with porous structure and small particle.

/
| 〈 |
|
〉 |