

FeNi-CoP/NC双功能催化剂的制备及电催化性能研究
收稿日期: 2021-11-18
修回日期: 2022-09-07
录用日期: 2022-09-10
网络出版日期: 2022-01-10
基金资助
国家自然科学基金项目(21975036);中央高校基本科研业务费专项(3132019328)
Preparation and Electrocatalytic Performance of FeNi-CoP/NC Bifunctional Catalyst
Received date: 2021-11-18
Revised date: 2022-09-07
Accepted date: 2022-09-10
Online published: 2022-01-10
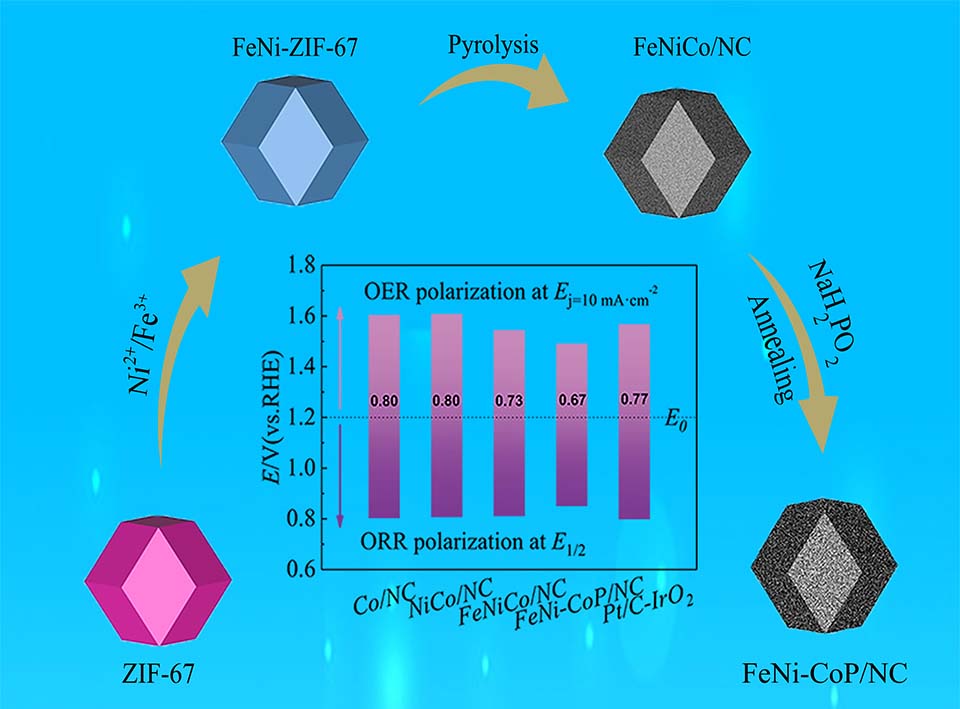
以ZIF-67为前驱体,采用异原子掺杂、高温热处理等方法制备了含有多种过渡金属、非金属粒子的多孔碳材料作为锌-空气电池催化剂。通过SEM、XRD、XPS和电化学方法对催化剂进行物理化学表征和催化性能测试,最后组装成全电池进行充放电性能实验。结果表明,制得的FeNi-CoP/NC的ORR半波电位达到了0.83 V,高于商用的Pt/C催化剂;OER电流密度在10 mA·cm-2时过电位为290 mV并可平稳地保持12 h,显示了良好的催化活性与稳定性。全电池性能测试显示其峰值功率密度较高为150 mW·cm-2,在3 mA·cm-2电流密度下保持了0.6 V的较窄电势间隙。
刘思淼 , 周景娇 , 季世军 , 文钟晟 . FeNi-CoP/NC双功能催化剂的制备及电催化性能研究[J]. 电化学, 2023 , 29(10) : 211118 . DOI: 10.13208/j.electrochem.211118
Rechargeable zinc-air batteries have gradually attracted much attention worldwide due to their high capacity, high energy density and low price. Oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) correspond to the charging and discharging processes in rechargeable zinc-air battery, respectively. At present, commercial Pt/C and IrO2 catalysts hinder the large-scale application of zinc-air batteries due to low reserves, high prices and poor stability. Therefore, exploring high performance, low cost and high stability with dual functional catalysts is important for the development of rechargeable zinc-Air batteries. The metal-organic frameworks (MOFs) have high specific surface area, structural stability, good catalytic activity and application prospects. Transition metals have high catalytic activity, but they are easily corroded in alkaline solutions. Non-metallic materials are inexpensive and have catalytic activity under a specific structure. Taking the advantages of the above-mentioned materials, ZIF-67 was used as the precursor, along with heteroatom doping and high temperature heat treatment to prepare a porous carbon material FeNi-CoP/NC containing multiple transition metals and non-metal particles as a zinc-air battery catalyst. Physical and chemical characterizations, and catalytic performance testing of the catalyst were carried out by SEM, XRD, XPS and electrochemical methods, and finally assembled into a full battery for charge and discharge performance experiments. The results showed that the prepared FeNi-CoP/NC catalyst had rhombohedral dodecahedron structure and specific surface area of 402 m2·g-1. The half-wave potential went up to 0.83 V when used as an electrocatalyst for oxygen reduction reaction in zinc-air batteries. After 5000 cycles, the current density only lost 5.06% and the half-wave potential changed little, revealing a good stability; the overpotential of OER was 290 mV at the current density of 10 mA·cm-2. And the catalyst could be kept stable for 12 h at 100 mA·cm-2. The performance test of the full battery demonstrated that the peak power density was as high as 150 mW·cm-2, and a narrow potential gap of 0.6 V was maintained at the current density of 3 mA·cm-2. The good catalytic activity might be mainly attributable to the fact that doping with multiple metal elements can provide rich valences to accelerate the four-step coordinated proton/electron transfer step, and the good conductivity of CoP also effectively improves the catalytic activity of FeNi-CoP/NC. This work provides useful guidance for improving the electrocatalytic performance of the catalyst through simple doping and heat treatment strategies.

/
| 〈 |
|
〉 |