

醇盐自模板法构筑碳封装NiFeV基电催化剂用于析氧反应
收稿日期: 2021-11-03
修回日期: 2021-12-01
录用日期: 2021-12-06
网络出版日期: 2021-12-19
基金资助
国家自然科学基金项目(91963109);中国博士后科学基金(2019M662583)
Constructing Carbon-Encapsulated NiFeV-Based Electrocatalysts by Alkoxide-Based Self-Template Method for Oxygen Evolution Reaction
Received date: 2021-11-03
Revised date: 2021-12-01
Accepted date: 2021-12-06
Online published: 2021-12-19
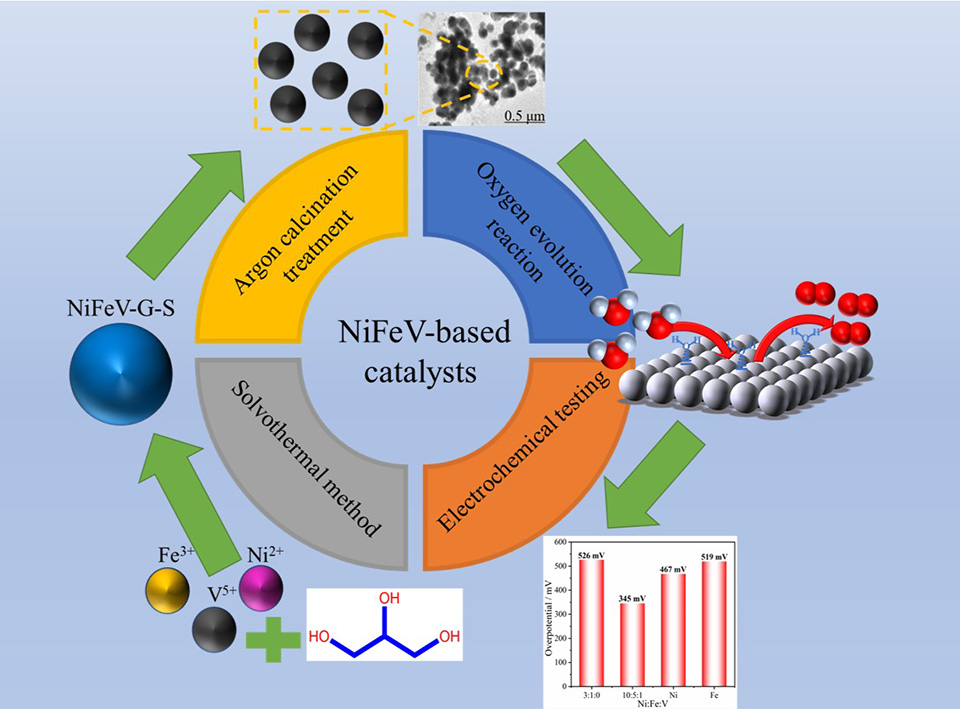
发展绿色可持续的水电解制氢技术有利于实现“碳中和”战略目标,而开发高效稳定的析氧反应催化剂对水电解技术至关重要。本研究以NiFeV固态金属醇盐为前驱体,采用醇盐自模板法制备碳封装NiFeV基催化剂。研究结果表明,NiFeV基催化剂呈现出均匀的球状结构,用于电解水析氧反应电催化剂时仅需381 mV的过电位即可获得20 mA·cm-2的电流密度。NiFeV基催化剂良好的催化活性和稳定性主要得益于均匀的球状结构,V对电子结构的优化调控以及封装碳层对金属颗粒的保护作用。此工作通过V掺杂和碳封装的策略,为提升析氧催化剂的电催化性能提供了有利借鉴。
马恩辉 , 刘旭坡 , 申涛 , 王得丽 . 醇盐自模板法构筑碳封装NiFeV基电催化剂用于析氧反应[J]. 电化学, 2023 , 29(11) : 211103 . DOI: 10.13208/j.electrochem.211103
The development of green and sustainable water-splitting hydrogen production technology is beneficial to reducing the over-reliance on fossil fuels and realizing the strategic goal of "carbon neutral". As one of the half reactions for water splitting, oxygen evolution reaction has suffered the problems of sluggish four-electron transfer process and relatively slow reaction kinetics. Therefore, exploring efficient and stable catalysts for oxygen evolution reaction is of critical importance for water-splitting technology. Metal alkoxides are a series of compounds formed by the coordination function of metal ions with alcohol molecules. Metal alkoxides possess the double advantages of organic materials and inorganic materials, which makes them reveal a promising application in the electrochemical field. In view of the poor activity and stability of the current oxygen evolution reaction electrocatalysts, this study has adopted the alkoxide-based self-template method to prepare the carbon-encapsulated NiFeV-based electrocatalysts through using the solid NiFeV-alkoxides as precursors. The organic components in solid metal alkoxides are employed to achieve the graphitized carbon encapsulation after the high-temperature calcination process, which is beneficial for improving the conductivity and corrosion resistance of catalysts. Through adjusting the V doping amounts and the calcination temperatures, the electronic structure of NiFe nanoparticles and carbon encapsulation were optimized, which are both key influence factors for oxygen evolution performances. As a result, the oxygen evolution catalysts with high activity and stability were obtained successfully in this work. The experimental results have shown that the NiFeV-based catalysts presented a uniform spherical structure with carbon encapsulation. The current density of 20 mA·cm-2 could be obtained at the overpotential of only 381 mV as an electrocatalyst for oxygen evolution reaction in water electrolysis. After the continuous 10000 s durability test, the NiFeV-based catalyst exhibited slight reduction in current density but still maintained the catalytic activity almost similar to the initial one, revealing a good oxygen evolution stability. The excellent catalytic activity and stability of NiFeV-based catalysts are believed to be mainly attributed to the uniform spherical structure, the optimized regulation of V on the electronic structure and the protective effect of carbon encapsulation on metal particles. The V element in the catalysts exhibited the rich redox states of V3+, V4+ and V5+, which can effectively adjust the electronic structure of adjacent atoms and optimize the binding energy of oxygen reduction reaction intermediates, thus improving the electrocatalytic performance of catalysts. This work provides a useful guidance for improving the electrocatalytic performance of oxygen evolution catalysts through the V-doping and carbon encapsulation strategies.

/
| 〈 |
|
〉 |