

同步辐射表征技术在金属空气电池研究中的应用
收稿日期: 2021-10-17
修回日期: 2021-12-04
网络出版日期: 2021-12-18
版权
Synchrotron X-Rays Characterizations of Metal-Air Batteries
Received date: 2021-10-17
Revised date: 2021-12-04
Online published: 2021-12-18
Copyright
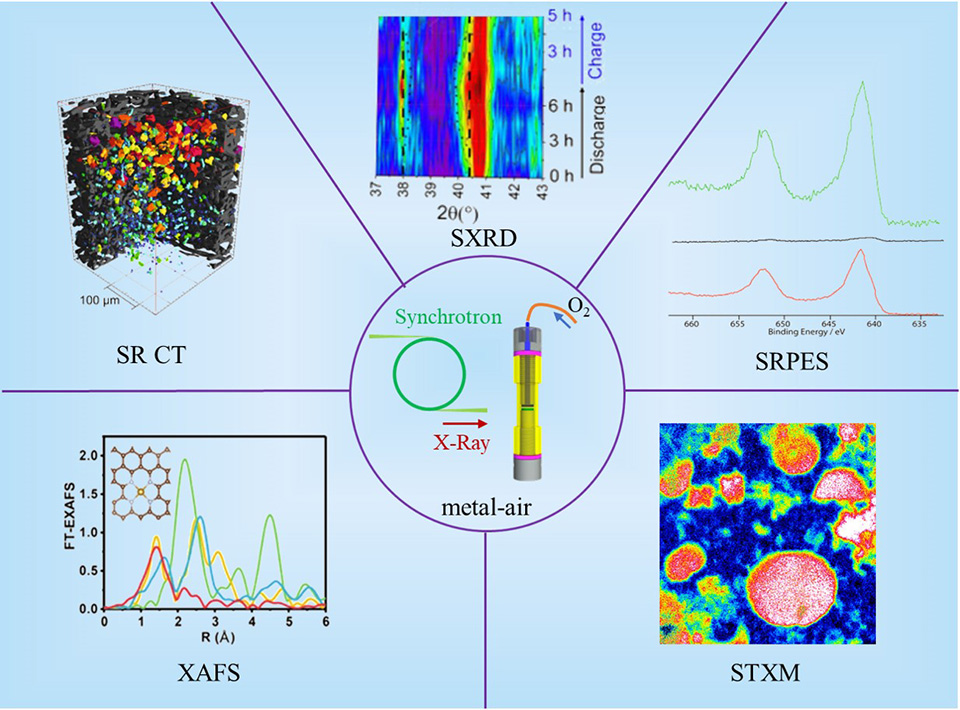
电动汽车的快速发展迫切需要高能量密度的电池。近年来,金属空气电池由于其超高的理论能量密度,在工业和学术领域引起了广泛的关注。然而,其副反应严重、能量效率低、循环寿命有限等诸多缺点严重阻碍了其实际应用的可行性。了解电池反应机理并进一步制定有效的策略有利于金属-空气电池的实际应用。在过去十年中,先进的表征技术加速了金属空气电池的发展。特别是基于同步加速器的表征技术因其无损检测能力和高分辨率已被广泛应用于金属空气电池的机理理解。在这篇综述中,我们系统地总结了各种用于分析金属空气电池局部结构和化学特性的同步辐射表征技术,特别关注于这些先进的表征技术如何帮助理解电池降解机理和优化策略的本质。本进展报告旨在强调同步辐射表征在金属空气电池机理理解的关键作用。
宋亚杰 , 孙雪 , 任丽萍 , 赵雷 , 孔凡鹏 , 王家钧 . 同步辐射表征技术在金属空气电池研究中的应用[J]. 电化学, 2022 , 28(3) : 2108461 . DOI: 10.13208/j.electrochem.210846
The rapid development of electric vehicles urgently requires high-energy-density batteries. Recently, metal-air batteries have attracted much attention in industry and academia for their ultra-high theoretical energy densities. However, the practical application of metal-air batteries is severely impeded by multiple drawbacks, including severe side reactions, low energy efficiency, and limited cycle life. Understanding the reaction mechanism of the cell and further developing effective strategies are beneficial for the practical application of metal-air batteries. In the past decade, advanced characterization techniques have accelerated the development of metal-air batteries. In particular, synchrotron radiation-based characterization techniques have been widely applied to the mechanistic study of metal-air batteries due to their non-destructive detection capability and high resolution. In this review, various synchrotron radiation-based characterization techniques are systematically summarized to understand the local structure and chemistry of metal-air batteries, with a special focus on how these advanced techniques can help understand the essence of degradation mechanism and optimization strategies. This progress report aims to highlight the crucial role of synchrotron radiation characterization for mechanism understanding of metal-air batteries.

/
| 〈 |
|
〉 |