

碳层网络促进Sn/SnO2纳米颗粒选择性CO2还原
收稿日期: 2021-10-06
修回日期: 2021-10-18
网络出版日期: 2021-10-21
版权
Selective CO2 Reduction to Formate on Heterostructured Sn/SnO2 Nanoparticles Promoted by Carbon Layer Networks
Received date: 2021-10-06
Revised date: 2021-10-18
Online published: 2021-10-21
Copyright
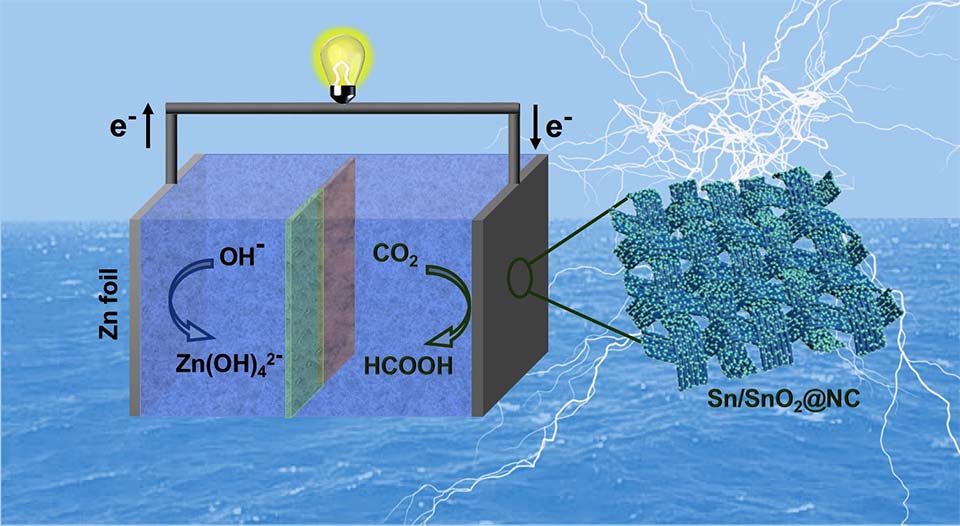
在各类CO2还原电催化剂中,锡基材料获得了研究人员的广泛关注,但其总体催化性能仍然受催化剂电极的组成,形貌和结构的限制。在本研究中,我们利用Sn低熔点(m.p. 232oC)的特性,在聚多巴胺碳化的同时实现Sn的熔化与再结晶,合成了由氮掺杂碳层网络分散的异质结构Sn/SnO2纳米颗粒自支撑电极(Sn/SnO2@NC)。氮掺杂碳层网络有利于电子的富集,可提高催化剂电极的导电性,防止超细纳米粒子的团聚,并保护其不在电解液中溶解。在CO2饱和的0.5 mol·L-1 NaHCO3水溶液中,所制备Sn/SnO2@NC电极与没有碳层网络包覆的电极相比,其CO2还原催化性能得到了很大的提高。该Sn/SnO2@NC电极在-0.9 V(vs. RHE)的电解电压下,电流密度为17 mA·cm-2,甲酸盐产物的选择性为83%。通过偶联该CO2还原催化电极与商品化RuO2催化剂作为水氧化阳极,可实现持续的CO2/H2O电解。此外,以Sn/SnO2@NC为阴极,Zn箔为阳极,我们还构建了可充放电的水系Zn-CO2电池。该电池的输出开路电压为1.35 V,峰值功率密度为0.9 mW·cm-2。本研究为高性能CO2还原催化剂的设计提供了新的思路,同时可充放电Zn-CO2电池的构建为绿色能源转换和存储系统提供了新的方案。
滕雪 , 牛艳丽 , 巩帅奇 , 刘璇 , 陈作锋 . 碳层网络促进Sn/SnO2纳米颗粒选择性CO2还原[J]. 电化学, 2022 , 28(2) : 2108441 . DOI: 10.13208/j.electrochem.210844
Tin (Sn)-based materials have emerged as promising electrocatalysts for selective reduction of CO2 to formate, but their overall performances are still limited by electrode structures which govern the accessibility to active sites, the electron transfer kinetics, and the catalytic stability. In this study, the heterostructured Sn/SnO2 nanoparticles dispersed by N-doped carbon layer networks (Sn/SnO2@NC) were synthesized by a melt-recrystallization method taking the low melting point of Sn (m.p. 232oC). The N-doped carbon layer networks derived from polydopamine could attract more electrons on the electrocatalyst, serve as conductive agents and protect the ultrafine nanoparticles from agglomeration and dissolution. The Sn/SnO2@NC electrode exhibited the greatly enhanced performance for CO2 reduction to formate in CO2-saturated 0.5 mol·L-1 aqueous NaHCO3 solution, showing a selectivity of 83% at only -0.9 V vs. RHE with a sustained current density of 17 mA·cm-2 for extended periods. By coupling the catalytic electrode with a commercially available RuO2 catalyst as the anode, the long-term CO2/H2O splitting has been achieved. Furthermore, a rechargeable aqueous Zn-CO2 battery with Sn/SnO2@NC as the cathode and Zn foil as the anode was constructed. It could output electric energy with an open circuit voltage of 1.35 V and a peak power density of 0.9 mW·cm-2.

/
| 〈 |
|
〉 |