

质子交换膜燃料电池铂基催化剂研究进展
收稿日期: 2021-08-05
修回日期: 2021-09-03
网络出版日期: 2021-09-17
基金资助
国家自然科学基金项目(21805121);云南省基础研究计划项目(2019FD137)
版权
Progress of Pt-Based Catalysts in Proton-Exchange Membrane Fuel Cells: A Review
Received date: 2021-08-05
Revised date: 2021-09-03
Online published: 2021-09-17
Copyright
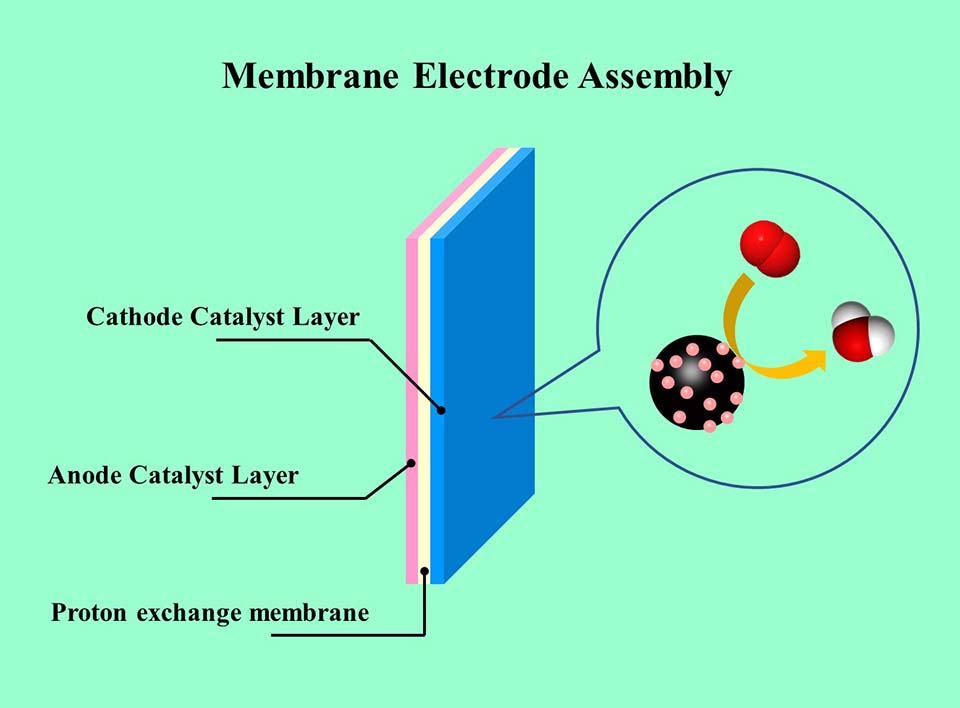
燃料电池是一种将化学能直接转化为电能的能量转换装置,具有能量密度高、利用率高、清洁安静等优点。在不同类型的燃料电池中,质子交换膜燃料电池(PEMFC)不仅能量密度高,而且具有在近常温条件下工作的特点,因此受到广泛关注。目前,商业化PEMFC仍采用铂基纳米材料作为催化剂,其中缺乏低成本、高效的阴极催化剂是限制PEMFC性能提升和成本降低的关键因素之一。本文综述PEMFC催化剂的结构可控制备及其对阴极氧还原反应和膜电极性能的影响,阐述调控催化剂结构提高PEMFC性能的方法,特别是提高贵金属催化剂的利用率,降低膜电极中贵金属用量的研究进展。
黄龙 , 徐海超 , 荆碧 , 李秋霞 , 易伟 , 孙世刚 . 质子交换膜燃料电池铂基催化剂研究进展[J]. 电化学, 2022 , 28(1) : 2108061 . DOI: 10.13208/j.electrochem.210806
Fuel cells are energy conversion devices that convert chemical energy directly into electricity. It has the advantages of high energy density, high utilization efficiency of fuel, clean and noiseless during working. Among all kinds of fuel cells, proton exchange membrane fuel cells (PEMFCs) are most popular since PEMFCs function at near ambient temperature, while their power densities are higher than those of other fuel cells. Currently, Pt-based nanomaterials are still the unreplaceable catalysts in commercialized PEMFCs. The lack of low-cost and high-performance cathode catalysts is still one of key factors that hampers the commercialization of PEMFCs. In this review, the structurally controlled syntheses of catalysts and their influences on the performances of oxygen reduction reaction (ORR) and membrane electrode assembly (MEA) are summarized. The performance of membrane electrode assembly (MEA) can also be adjusted by regulating the structure of catalyst layer. Special attention has been paid with a focus on the achievement of enhanced utilization of noble metal, and thus, lowering the loading of noble metals in MEA.

| [1] | Zhang J. PEM fuel cell electrocatalysts and catalyst layers: fundamentals and applications[M]. 2008, London: Springer. |
| [2] | Debe M K. Electrocatalyst approaches and challenges for automotive fuel cells[J]. Nature, 2012, 486(7401): 43-51. |
| [3] | Fuel Cell System Cost-2015 [2021-08-05][EB/OL]. https://www.hydrogen.energy.gov/pdfs/15015_fuel_cell_system_cost_2015.pdf |
| [4] | Van Zee J W. Proceedings of the symposium on diaphragms, separators, and ion-exchange membranes[J]. 1986. Electrochemical Society. |
| [5] | Petrow H G, Allen R J. Finely particulated colloidal platinum compound and sol for producing the same, and method of preparation[P]. United States Patent, 3992512, 1976-11-16. |
| [6] | Román-Martínez M C, Cazorla-Amorós D, Linares-Solano A, Delecea C S M, Yamashita H, Anpo M. Metal-support interaction in Pt/C catalysts. Influence of the support surface chemistry and the metal precursor[J]. Carbon, 1995, 33(1): 3-13. |
| [7] | Amine K, Mizuhata M, Oguro K, Takenaka H. Catalytic activity of platinum after exchange with surface active functional groups of carbon blacks[J]. J. Chem. Soc., Faraday Trans, 1995, 91(24): 4451-4458. |
| [8] | Zeng J H, Lee J Y, Zhou W. Activities of Pt/C catalysts prepared by low temperature chemical reduction methods[J]. Appl. Catal. A - Gen., 2006, 308: 99-104. |
| [9] | Kim M, Park J N, Kim H, Song S, Lee W H. The preparation of Pt/C catalysts using various carbon materials for the cathode of PEMFC[J]. J. Power Sources, 2006, 163(1): 93-97. |
| [10] | Yu W Y, Tu W X, Liu H F. Synjournal of nanoscale platinum colloids by microwave dielectric heating[J]. Langmuir, 1999, 15(1): 6-9. |
| [11] | Li H Q, Xin Q, Li W Z, Zhou Z H, Jiang L H, Yang S H, Sun G Q. An improved palladium-based DMFCs cathode catalyst[J]. Chem. Commun., 2004, 23: 2776-2777. |
| [12] | Zhou Z H, Wang S L, Zhou W J, Wang G X, Jiang L H, Li W Z, Song S Q, Liu J G, Sun G Q, Xin Q. Novel synjournal of highly active Pt/C cathode electrocatalyst for direct methanol fuel cell[J]. Chem. Commun., 2003, 3: 394-395. |
| [13] | Pak Hoe L, Boaventura M, Lagarteira T, Kee Shyuan L, Mendes A. Polyol synjournal of reduced graphene oxide supported platinum electrocatalysts for fuel cells: Effect of Pt precursor, support oxidation level and pH[J]. Int. J. Hydrogen Energy, 2018, 43(35): 16998-17011. |
| [14] | Wang Y J, Zhao N, Fang B, Li H, Bi X T, Wang H. Effect of different solvent ratio (ethylene glycol/water) on the preparation of Pt/C catalyst and its activity toward oxygen reduction reaction[J]. RSC Advances, 2015, 5(70): 56570-56577. |
| [15] | Lee W D, Lim D H, Chun H J, Lee H I. Preparation of Pt nanoparticles on carbon support using modified polyol reduction for low-temperature fuel cells[J]. Int. J. Hydrogen Energy, 2012, 37(17): 12629-12638. |
| [16] | Li Y X, Zhang Z Y, Xiao Z J, Zhao G Z, Song H Y, Liu Y Q, Zeng J H. Stable and active Pt colloid preparation by modified citrate reduction and a mechanism analysis of inorganic additives[J]. J. Colloid Interface Sci., 2020, 572: 74-82. |
| [17] | Quinson J, Inaba M, Neumann S, Swane A A, Bucher J, Simonsen S B, Theil Kuhn L, Kirkensgaard J J K, Jensen K M Ø, Oezaslan M, Kunz S, Arenz M. Investigating particle size effects in catalysis by applying a size-controlled and surfactant-free synjournal of colloidal nanoparticles in alkaline ethylene glycol: case study of the oxygen reduction reaction on Pt[J]. ACS Catalysis, 2018, 8(7): 6627-6635. |
| [18] | Zhao J, Chen W X, Zheng Y F, Li X, Xu Z D. Microwave polyol synjournal of Pt/C catalysts with size-controlled Pt particles for methanol electrocatalytic oxidation[J]. J. Mater. Sci., 2006, 41(17): 5514-5518. |
| [19] | Zhang L M, Wang Z B, Zhang J J, Sui X L, Zhao L, Han J C, Investigation on electrocatalytic activity and stability of Pt/C catalyst prepared by facile solvothermal synjournal for direct methanol fuel cell[J]. Fuel Cells, 2015, 15(4): 619-627. |
| [20] | Friebel D, Viswanathan V, Miller D J, Anniyev T, Ogasawara H, Larsen A H, O’Grady C P, Nørskov J K, Nilsson A. Balance of nanostructure and bimetallic interactions in Pt model fuel cell catalysts: In situ XAS and DFT study[J]. J. Am. Chem. Soc., 2012, 134(23): 9664-9671. |
| [21] | Escudero-Escribano M, Malacrida P, Hansen M H, Vej-Hansen U G, Velázquez-Palenzuela A, Tripkovic V, Sch-iøtz J, Rossmeisl J, Stephens I E L, Chorkendorff I. Tuning the activity of Pt alloy electrocatalysts by means of the lanthanide contraction[J]. Science, 2016, 352(6281): 73-76. |
| [22] | Carpenter M K, Moylan T E, Kukreja R S, Atwan M H, Tessema M M. Solvothermal synjournal of platinum alloy nanoparticles for oxygen reduction electrocatalysis[J]. J. Am. Chem. Soc., 2012, 134(20): 8535-8542. |
| [23] | Cheng Z Z, Liao S, Zhou W P, Luo G S, Huang H F. Straightforward synjournal of chemically ordered Pt3Co/C nanoparticles by a solid phase method for oxygen-reduction reaction[J]. Ionics, 2021, 27(6): 2553-2560. |
| [24] | Leteba G M, Wang Y C, Slater T J A, Cai R, Byrne C, Race C P, Mitchell D R G, Levecque P B J, Young N P, Holmes S M, Walton A, Kirkland A I, Haigh S J, Lang C I. Oleylamine aging of PtNi nanoparticles giving enhanced functionality for the oxygen reduction reaction[J]. Nano Lett., 2021, 21(9): 3989-3996. |
| [25] | Wang D, Xin H L, Hovden R, Wang H, Yu Y, Muller D A, DiSalvo F J, Abruña H D. Structurally ordered intermetallic platinum-cobalt core-shell nanoparticles with enhanced activity and stability as oxygen reduction electrocatalysts[J]. Nat. Mater., 2013, 12(1): 81-87. |
| [26] | Strasser P, Koh S, Anniyev T, Greeley J, More K, Yu C, Liu Z, Kaya S, Nordlund D, Ogasawara H, Toney M F, Nilsson A. Lattice-strain control of the activity in dealloyed core-shell fuel cell catalysts[J]. Nat. Chem., 2010, 2(6): 454-460. |
| [27] | Alinezhad A, Benedetti T M, Gloag L, Cheong S, Watt J, Chen H S, Gooding J J, Tilley R D. Controlling Pt crystal defects on the surface of Ni-Pt core-shell nanoparticles for active and stable electrocatalysts for oxygen reduction[J]. ACS Appl. Nano Mater., 2020, 3(6): 5995-6000. |
| [28] | Tao L, Huang B L, Jin F D, Yang Y, Luo M C, Sun M Z, Liu Q, Gao F M, Guo S J. Atomic PdAu interlayer sandwiched into Pd/Pt core/shell nanowires achieves superstable oxygen reduction catalysis[J]. ACS Nano, 2020, 14(9): 11570-11578. |
| [29] | Zhang Y F, Qin J, Leng D Y, Liu Q R, Xu X Y, Yang B, Yin F. Tunable strain drives the activity enhancement for oxygen reduction reaction on Pd@Pt core-shell electrocatalysts[J]. J. Power Sources, 2021, 485: 229340. |
| [30] | Sasaki K, Kuttiyiel K A, Adzic R R. Designing high performance Pt monolayer core-shell electrocatalysts for fuel cells[J]. Curr. Opin. Electrochem., 2020, 21: 368-375. |
| [31] | Zhao Y P, Tao L, Dang W, Wang L L, Xia M R, Wang B, Liu M M, Gao F M, Zhang J J, Zhao Y F. High-indexed PtNi alloy skin spiraled on Pd nanowires for highly efficient oxygen reduction reaction catalysis[J]. Small, 2019, 15(17): 1900288. |
| [32] | Jang Y, Choi K H, Chung D Y, Lee J E, Jung N, Sung Y E. Self-assembled dendritic Pt nanostructure with high-index facets as highly active and durable electrocatalyst for oxygen reduction[J]. ChemSusChem, 2017, 10(15): 3063-3068. |
| [33] | Chen C, Kang Y J, Huo Z Y, Zhu Z W, Huang W Y, Xin H L, Snyder J D, Li D G, Herron J A, Mavrikakis M, Chi M F, More K L, Li Y D, Markovic N M, Somorjai G A, Yang P D, Stamenkovic V R. Highly crystalline multimetallic nanoframes with three-dimensional electrocatalytic surfaces[J]. Science, 2014, 343(6177): 1339-1343. |
| [34] | Chen S P, Li M F, Gao M Y, Jin J B, van Spronsen M A, Salmeron M B, Yang P D. High-performance Pt-Co nano-frames for fuel-cell electrocatalysis[J]. Nano Lett., 2020, 20(3): 1974-1979. |
| [35] | Banham D, Ye S. Current status and future development of catalyst materials and catalyst layers for proton exchange membrane fuel cells: an industrial perspective[J]. ACS Energy Lett., 2017, 2(3): 629-638. |
| [36] | Koh S, Toney M F, Strasser P. Activity-stability relationships of ordered and disordered alloy phases of Pt3Co electrocatalysts for the oxygen reduction reaction (ORR)[J]. Electrochim. Acta, 2007, 52(8): 2765-2774. |
| [37] | Mani P, Srivastava R, Strasser P. Dealloyed binary PtM3 (M = Cu, Co, Ni) and ternary PtNi3M (M=Cu, Co, Fe, Cr) electrocatalysts for the oxygen reduction reaction: Performance in polymer electrolyte membrane fuel cells[J]. J Power Sources, 2011, 196(2): 666-673. |
| [38] | Liu M Y, Hu A P, Ma Y N, Wang G L, Zou L L, Chen X H, Yang H, Nitrogen-doped Pt3Co intermetallic compound nanoparticles: A durable oxygen reduction electrocatalyst[J]. J. Electroanal. Chem., 2020, 871: 114267. |
| [39] | Yoo T Y, Yoo J M, Sinha A K, Bootharaju M S, Jung E, Lee H S, Lee B H, Kim J, Antink W H, Kim Y M, Lee J, Lee E, Lee D W, Cho S P, Yoo S J, Sung Y E, Hyeon T. Direct synjournal of intermetallic platinum-alloy nanoparticles Highly loaded on carbon supports for efficient electrocatalysis[J]. J. Am. Chem. Soc., 2020, 142(33): 14190-14200. |
| [40] | Wang F, Zhang Q, Rui Z Y, Li J, Liu J G. High-loading Pt-Co/C catalyst with enhanced durability toward the oxygen reduction reaction through surface Au modification[J]. ACS Appl. Mater. Inter., 2020, 12(27): 30381-30389. |
| [41] | Zhang M Y, Shi J J, Ning W S, Hou Z Y. Reduced gra-phene oxide decorated with PtCo bimetallic nanoparticles: Facile fabrication and application for base-free oxidation of glycerol[J]. Catal. Today, 2017, 298(SI): 234-240. |
| [42] | Li J R, Sharma S, Liu X M, Pan Y T, Spendelow J S, Chi M F, Jia Y K, Zhang P, Cullen D A, Xi Z, Lin H H, Yin Z Y, Shen B, Muzzio M, Yu C, Kim Y S, Peterson A A, More K L, Zhu H Y, Sun S H. Hard-magnet L10-CoPt nanoparticles advance fuel cell catalysis[J]. Joule, 2019, 3(1): 124-135. |
| [43] | Tian X, Zhao X, Su Y Q, Wang L, Wang H, Dang D, Chi B, Liu H, Hensen E J M, Lou X W, Xia B Y. Engineering bunched Pt-Ni alloy nanocages for efficient oxygen reduction in practical fuel cells[J]. Science, 2019, 366(6467): 850-856. |
| [44] | Cui C, Gan L, Li H H, Yu S H, Heggen M, Strasser P. Octahedral PtNi nanoparticle catalysts: exceptional oxygen reduction activity by tuning the alloy particle surface composition[J]. Nano Lett., 2012, 12(11): 5885-5889. |
| [45] | Cui C, Gan L, Heggen M, Rudi S, Strasser P. Compositional segregation in shaped Pt alloy nanoparticles and their structural behaviour during electrocatalysis[J]. Nat. Mater., 2013, 12(8): 765-771. |
| [46] | Chung Y H, Chung D Y, Jung N, Park H Y, Yoo S J, Jang J H, Sung Y E. Origin of the enhanced electrocatalysis for thermally controlled nanostructure of bimetallic nanoparticles[J]. J. Phys. Chem. C, 2014, 118(19): 9939-9945. |
| [47] | Huang X Q, Zhao Z P, Chen Y, Zhu E B, Li M F, Duan X F, Huang Y. A rational design of carbon-supported dispersive Pt-based octahedra as efficient oxygen reduction reaction catalysts[J]. Energy Environ. Sci., 2014, 7(9): 2957-2962. |
| [48] | Dionigi F, Weber C C, Primbs M, Gocyla M, Bonastre A M, Spöri C, Schmies H, Hornberger E, Kühl S, Drnec J, Heggen M, Sharman J, Dunin-Borkowski R E, Strasser P. Controlling near-surface Ni composition in octahedral PtNi(Mo) nanoparticles by Mo doping for a highly active oxygen reduction reaction catalyst[J]. Nano Lett., 2019, 19(10): 6876-6885. |
| [49] | Lim J, Shin H, Kim M, Lee H, Lee K S, Kwon Y, Song D, Oh S, Kim H, Cho E. Ga-doped Pt-Ni octahedral nano-particles as a highly active and durable electrocatalyst for oxygen reduction reaction[J]. Nano Lett., 2018, 18(4): 2450-2458. |
| [50] | Huang X Q, Zhao Z P, Cao L, Chen Y, Zhu E B, Lin Z Y, Li M F, Yan A M, Zettl A, Wang Y M, Duan X F, Mueller T, Huang Y. High-performance transition metal-doped Pt3Ni octahedra for oxygen reduction reaction[J]. Science, 2015, 348(6240): 1230-1234. |
| [51] | Arán-Ais R M, Dionigi F, Merzdorf T, Gocyla M, Heggen M, Dunin-Borkowski R E, Gliech M, Solla-Gullón J, Herrero E, Feliu J M, Strasser P. Elemental anisotropic growth and atomic-scale structure of shape-controlled octahedral Pt-Ni-Co alloy nanocatalysts[J]. Nano Lett., 2015, 15(11): 7473-7480. |
| [52] | Uskokovic V, Drofenik M. Reverse micelles: Inert nano-reactors or physico-chemically active guides of the capped reactions[J]. Adv. Colloid Interface Sci., 2007, 133(1): 23-34. |
| [53] | Bedia J, Lemus J, Calvo L, Rodriguez J J, Gilarranz M A. Effect of the operating conditions on the colloidal and microemulsion synjournal of Pt in aqueous phase[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2017, 525: 77-84. |
| [54] | Eriksson S, Nylén U, Rojas S, Boutonnet M. Preparation of catalysts from microemulsions and their applications in heterogeneous catalysis[J]. Appl. Catal. A-Gen., 2004, 265(2): 207-219. |
| [55] | Watanabe M, Yano H, Tryk D A, Uchida H. Highly durable and active PtCo alloy/graphitized carbon black cathode catalysts by controlled deposition of stabilized Pt skin layers[J]. J. Electrochem. Soc., 2016, 163(6): F455-F463. |
| [56] | Jayabal S, Saranya G, Geng D, Lin L Y, Meng X. Insight into the correlation of Pt-support interactions with electrocatalytic activity and durability in fuel cells[J]. J. Mater. Chem. A, 2020, 8(19): 9420-9446. |
| [57] | Li H, Chen C, Yan D F, Wang Y Y, Chen R, Zou Y Q, Wang S Y. Interfacial effects in supported catalysts for electrocatalysis[J]. J. Mater. Chem. A, 2019, 7(41): 23432-23450. |
| [58] | Li Z, Song M, Zhu W Y, Zhuang W C, Du X H, Tian L. MOF-derived hollow heterostructures for advanced electrocatalysis[J]. Coord. Chem. Rev., 2021, 439: 213946. |
| [59] | Wang X X, Hwang S, Pan Y, Chen K, He Y, Karakalos S, Zhang H, Spendelow J S, Su D, Wu G. Ordered Pt3Co Intermetallic nanoparticles derived from metal-organic frameworks for oxygen reduction[J]. Nano Lett., 2018, 18(7): 4163-4171. |
| [60] | Chong L, Wen J, Kubal J, Sen F G, Zou J, Greeley J, Chan M, Barkholtz H, Ding W, Liu D J. Ultralow-loading platinum-cobalt fuel cell catalysts derived from imidazolate frameworks[J]. Science, 2018, 362(6420): 1276-1281. |
| [61] | Han B, Carlton C E, Kongkanand A, Kukreja R S, Theo-bald B R, Gan L, O'Malley R, Strasser P, Wagner F T, Shao-Horn Y. Record activity and stability of dealloyed bimetallic catalysts for proton exchange membrane fuel cells[J]. Energy Environ. Sci., 2015, 8(1): 258-266. |
| [62] | Xiao F, Qin X P, Xu M J, Zhu S Q, Zhang L L, Hong Y M, Choi S I, Chang Q W, Xu Y, Pan X Q, Shao M H. Impact of heat treatment on the electrochemical properties of carbon-supported octahedral Pt-Ni nanoparticles[J]. ACS Catal., 2019, 9(12): 11189-11198. |
| [63] | Kühl S, Gocyla M, Heyen H, Selve S, Heggen M, Dunin-Borkowski R E, Strasser P. Concave curvature facets benefit oxygen electroreduction catalysis on octahedral shaped PtNi nanocatalysts[J]. J. Mater. Chem. A, 2019, 7(3): 1149-1159. |
| [64] | Wang T Y, Liang J S, Zhao Z L, Li SZ, Lu G, Xia Z C, Wang C, Luo J H, Han J T, Ma C, Huang Y H, Li Q. Sub-6 nm fully ordered L10-Pt-Ni-Co nanoparticles enhance oxygen reduction via Co doping induced ferromagnetism enhancement and optimized surface strain[J]. Adv. Energy Mater., 2019, 9(17): 1803771. |
| [65] | Sun R L, Xia Z X, Xu X L, Deng R Y, Wang S L, Sun G Q. Periodic evolution of the ionomer/catalyst interfacial structures towards proton conductance and oxygen transport in polymer electrolyte membrane fuel cells[J]. Nano Energy, 2020, 75: 104919. |
| [66] | Uchida M, Aoyama Y, Eda N, Ohta A. New preparation method for polymer-electrolyte fuel cells[J]. J. Electrochem. Soc., 1995, 142(2): 463-468. |
| [67] | Shin S J, Lee J K, Ha H Y, Hong S A, Chun H S, Oh I H. Effect of the catalytic ink preparation method on the performance of polymer electrolyte membrane fuel cells[J]. J. Power Sources, 2002, 106(1): 146-152. |
| [68] | Van Cleve T, Khandavalli S, Chowdhury A, Medina S, Pylypenko S, Wang M, More K L, Kariuki N, Myers D J, Weber A Z, Mauger S A, Ulsh M, Neyerlin K C. Dictating Pt-based electrocatalyst performance in polymer electrolyte fuel cells, from formulation to application[J]. ACS Appl. Mater. Inter., 2019, 11(50): 46953-46964. |
| [69] | Xu F, Zhang H, Ilavsky J, Stanciu L, Ho D, Justice M J, Petrache H I, Xie J. Investigation of a catalyst ink dispersion using both ultra-small-angle X-ray scattering and cryogenic TEM[J]. Langmuir, 2010, 26(24): 19199-19208. |
| [70] | Takahashi S, Shimanuki J, Mashio T, Ohma A, Tohma H, Ishihara A, Ito Y, Nishino Y, Miyazawa A. Observation of ionomer in catalyst ink of polymer electrolyte fuel cell using cryogenic transmission electron microscopy[J]. Ele-ctrochim. Acta, 2017, 224: 178-185. |
| [71] | Balu R, Choudhury N R, Mata J P, de Campo L, Rehm C, Hill A J, Dutta N K. Evolution of the interfacial structure of a catalyst ink with the quality of the dispersing solvent: a contrast variation small-angle and ultrasmall-angle neutron scattering investigation[J]. ACS Appl. Mater. Inter., 2019, 11(10): 9934-9946. |
| [72] | Wang M, Park J H, Kabir S, Neyerlin K C, Kariuki N N, Lv H, Stamenkovic V R, Myers D J, Ulsh M, Mauger S A. Impact of catalyst ink dispersing methodology on fuel cell performance using in-situ X-ray scattering[J]. ACS Appl. Energy Mater., 2019, 2(9): 6417-6427. |
| [73] | Xue Q, Yang D J, Li B, Ming P W, Zhang C M. Quantitative analysis effect of the cathode catalyst layer with various ionomer ratio on PEMFC by protonic resistance[J]. ECS Trans., 2019, 89(7): 23-28. |
| [74] | Alink R, Singh R, Schneider P, Christmann K, Schall J, Keding R, Zamel N. Full parametric study of the influence of ionomer content, catalyst loading and catalyst type on oxygen and ion transport in PEM fuel cell catalyst layers[J]. Molecules, 2020, 25(7): 1523. |
| [75] | Shi Y, Lu Z X, Guo L L, Yan C F. Fabrication of membrane electrode assemblies by direct spray catalyst on water swollen Nafion membrane for PEM water electrolysis[J]. Int. J. Hydrogen Energy, 2017, 42(42): 26183-26191. |
| [76] | Sassin M B, Garsany Y, Gould B D, Swider-Lyons K E. Fabrication method for laboratory-scale high-performance membrane electrode assemblies for fuel cells[J]. Anal. Chem., 2017, 89(1): 511-518. |
| [77] | Vierrath S, Breitwieser M, Klingele M, Britton B, Holdcroft S, Zengerle R, Thiele S. The reasons for the high power density of fuel cells fabricated with directly deposited membranes[J]. J. Power Sources, 2016, 326: 170-175. |
| [78] | Wei Z X, Su K H, Sui S, He A, Du S F. High performance polymer electrolyte membrane fuel cells (PEMFCs) with gradient Pt nanowire cathodes prepared by decal transfer method[J]. Int. J. Hydrogen Energy, 2015, 40(7): 3068-3074. |
| [79] | Zheng Z F, Yang F, Lin C, Zhu F J, Shen S Y, Wei G H, Zhang J L. Design of gradient cathode catalyst layer (CCL) structure for mitigating Pt degradation in proton exchange membrane fuel cells (PEMFCs) using mathematical method[J]. J. Power Sources, 2020, 451: 227729. |
| [80] | Chen G Y, Wang C, Lei Y J, Zhang J, Mao Z, Mao Z Q, Guo J W, Li J, Ouyang M. Gradient design of Pt/C ratio and Nafion content in cathode catalyst layer of PEMFCs[J]. Int. J. Hydrogen Energy, 2017, 42(50): 29960-29965. |
| [81] | Middelman E. Improved PEM fuel cell electrodes by controlled self-assembly[J]. Fuel Cells Bulletin, 2002, 11: 9-12. |
| [82] | Chen M, Wang M, Yang Z Y, Wang X D. High performance and durability of order-structured cathode catalyst layer based on TiO2@PANI core-shell nanowire arrays[J]. Appl. Surf. Sci., 2017, 406: 69-76. |
| [83] | Zeng Y C, Zhang H J, Wang Z Q, Jia J, Miao S, Song W, Xiao Y, Yu H M, Shao Z G, Yi B L. Nano-engineering of a 3D-ordered membrane electrode assembly with ultrathin Pt skin on open-walled PdCo nanotube arrays for fuel cells[J]. J. Mater. Chem. A, 2018, 6(15): 6521-6533. |
| [84] | Debe M K. Tutorial on the fundamental characteristics and practical properties of nanostructured thin film (NSTF) catalysts[J]. J. Electrochem. Soc., 2013, 160(6): F522-F534. |
/
| 〈 |
|
〉 |