

Pt@BaSrTiO3纳米材料的制备及其光电化学合成氨性能的研究
收稿日期: 2021-06-16
修回日期: 2021-08-13
网络出版日期: 2021-08-24
基金资助
国家自然科学基金项目(21972027)
Preparation of Pt@BaSrTiO3 Nanostructure and Its Properties towards Photoelectrochemical Ammonia Synthesis
Received date: 2021-06-16
Revised date: 2021-08-13
Online published: 2021-08-24
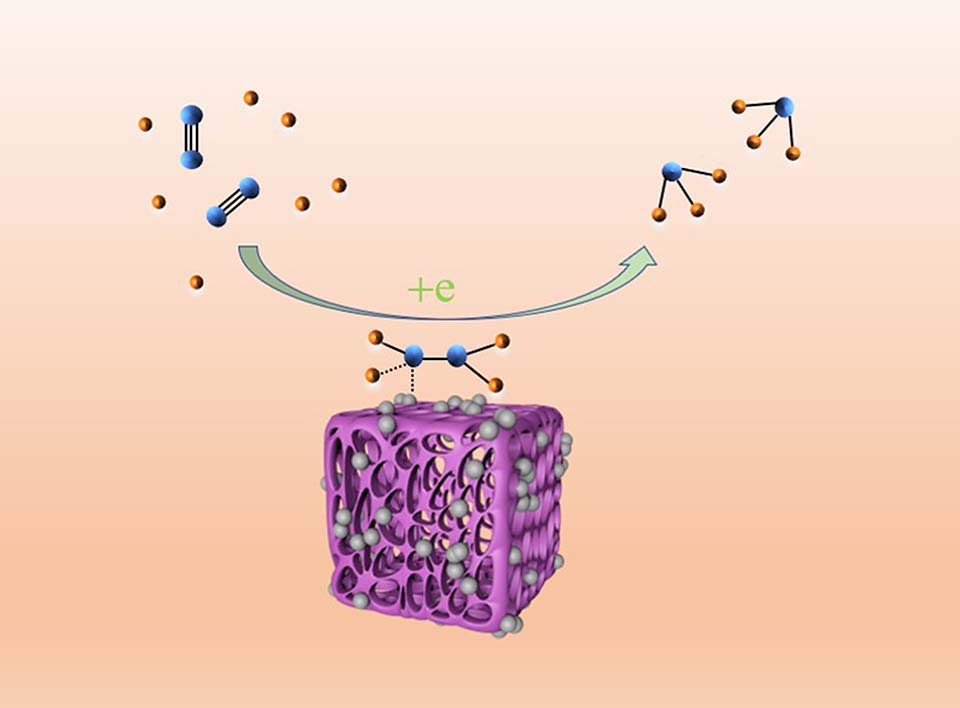
氨是一种重要的工业原料,也是一种潜在的绿色能源,在环境条件下利用可再生能源将氮气还原合成氨是一种有吸引力的方法,但是开发高效光电化学合成氨的催化剂仍然是一个挑战。在此,采用两步法制备BaSrTiO3,并用管式炉煅烧将Pt纳米颗粒修饰在BaSrTiO3上制备Pt@BaSrTiO3。采用XRD、BET、XPS、SEM、TEM、UV-Vis和PL进行表征,分析所得催化剂的晶体结构、形貌和光学性能。研究BaSrTiO3和Pt@BaSrTiO3在常温常压下光电化学合成氨性能。结果表明Pt@BaSrTiO3在-0.3 V(vs. RHE)电位下合成氨的产率为26.57 × 10-8 mol·h-1·mg-1,是纯BaSrTiO3的2倍(13.12 × 10-8 mol·h-1·mg-1)。另外,Pt@BaSrTiO3作为催化剂在-0.3 V(vs. RHE)的法拉第效率为5.43%。通过在BaSrTiO3上修饰Pt,增加催化剂的活性位点。修饰Pt减小催化剂的带隙,可见光吸收范围增大。此外, Pt-BST异质结结构进一步增强电荷分离和转移,抑制电子-空穴对的复合从而提高电荷分离效率。这项工作为进一步设计钙钛矿催化剂来提高氨的产率提供了良好的思路。
张静 , 郭瑞霞 , 浮建军 , 尹诗斌 , 沈培康 , 张信义 . Pt@BaSrTiO3纳米材料的制备及其光电化学合成氨性能的研究[J]. 电化学, 2022 , 28(4) : 2106161 . DOI: 10.13208/j.electrochem.210616
Ammonia is an important industrial raw material and a potential green energy. Using renewable energy to convert nitrogen into ammonia under ambient condition is an attractive method. However, the development of efficient photoelectrochemical ammonia synthesis catalysts remains a challenge. Perovskite such as BaSrTiO3 (BST) is a good photocatalytic material. However, BST is active under ultraviolet light and has a high recombination rate of photogenerated electron-hole pairs. By dispersing precious metals, it can effectively regulate the absorption of sunlight by BST. In this work, we used a two-step method to prepare BST. The H2PtCl6·6H2O solution was dispersed on the BST, and then followed by calcination in a tube furnace to obtain Pt@BaSrTiO3 (Pt@BST). X-ray powder diffraction (XRD), X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM), and transmission electron microscopy (TEM) were utilized to analyze the structures, morphologies, and surface chemical composition of the synthesized materials. Results showed that the well-crystallized Pt particles were successfully loaded onto the BST surface, and Pt and BST interacted to produce a metal-semiconductor heterojunction, improving the performance of N2 reduction. The N2 adsorption and desorption isotherms showed that the increase in the specific surface area helped the catalyst to adsorb N2, and the contact area with H2O also increased, which promotes the occurrence of NRR and thus produces more NH3. UV-Vis and PL spectroscopic techniques were used to characterize and analyze optical properties of the obtained catalyst. It is indicated that decoration of Pt reduces the band gap of the catalyst and increases the visible light absorption range, in addition, further enhances the charge separation and transfer, inhibits the recombination of electron-hole pairs, and improves the efficiency of charge separation. The performances of BST and Pt@BST for photoelectric catalytic synthesis of ammonia under ambient condition were studied. The yield of ammonia first increased and then decreased with the increase of Pt content. When the Pt content was 4wt%, the yield was the highest. The results showed that the ammonia yield of Pt@BST was 26.57 × 10-8 mol·h-1·mg-1 and Faraday efficiency (FE) was 5.43% at -0.3 V (vs. RHE) in 0.1 mol·L-1 Na2SO4 under natural conditions, suggesting that the ammonia yield of Pt@BST was twice that of pure BST (13.12 × 10-8 mol·h-1·mg-1). We conducted control experiments of 15N2 isotope and Ar in order to eliminate internal and external environmental pollution. Confirming that the detected NH3 was produced exclusively via nitrogen reduction reaction. After recycling the test six times at -0.3 V (vs. RHE), both FE and ammonia yield rate showed a slight variation, indicating the high stability of Pt@BST during N2 reduction process. This work provides a simple strategy for further designing the preparation of noble metal modified perovskite catalysts, and has promising application prospects in ammonia synthesis under ambient condition.

Key words: ammonia synthesis; nitrogen reduction; Pt; BaSrTiO3
/
| 〈 |
|
〉 |