

电化学合成乙酰基吡嗪
收稿日期: 2021-07-05
修回日期: 2021-08-06
网络出版日期: 2021-08-17
基金资助
国家重点研发计划项目(2017YFB0307502)
Electrochemical Synthesis of Acetylpyrazine
Received date: 2021-07-05
Revised date: 2021-08-06
Online published: 2021-08-17
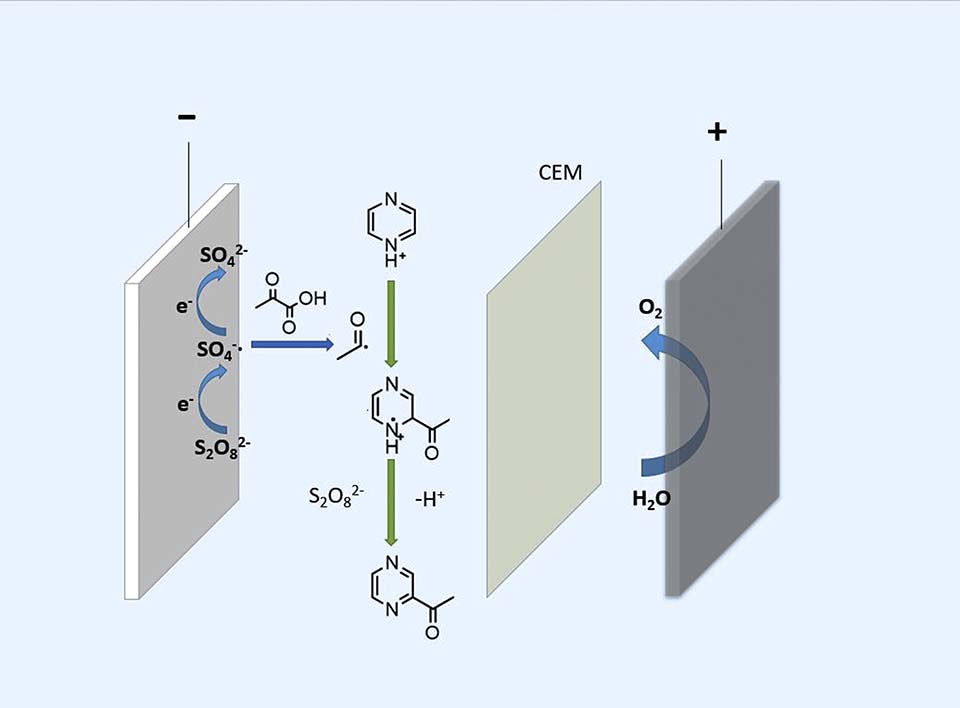
本文以吡嗪和丙酮酸为原料,在铅电极上电化学活化过硫酸铵得到的硫酸根自由基为氧化剂,首次采用电化学方法合成了乙酰基吡嗪。探究了电流密度、反应物摩尔比、反应物浓度、过硫酸铵、 pH值对乙酰基吡嗪收率的影响,同时在外加硫酸亚铁的条件下探究复合活化法对收率的影响。在最优条件下(电流密度100 A·m-2,丙酮酸浓度0.33 mol·L-1,吡嗪浓度1.00 mol·L-1),该反应的收率为44.12%。该工艺反应条件温和,简单易控,利用“清洁能源”电子代替了过渡金属盐以活化过硫酸铵,因而是一种环境友好的乙酰基吡嗪制备方法,具有广阔的工业化应用前景。
关键词: Minisci酰基化反应; 乙酰基吡嗪; 电化学还原; 过硫酸铵; 硫酸根自由基
毛麟 , 钮东方 , 胡硕真 , 张新胜 . 电化学合成乙酰基吡嗪[J]. 电化学, 2022 , 28(5) : 2107061 . DOI: 10.13208/j.electrochem.210706
Acetylpyrazine is naturally presented in hazelnuts, peanuts, and sesame seeds. As an important food additive, it is widely used in baked foods, meat, sesame and tobacco. At the same time, acetylpyrazine is also an important pharmaceutical intermediate, which is used in the syntheses of anti-tuberculosis drugs, anti-tumor, anti-malaria, anti-viral, antibacterial and treatments of epilepsy, pain and Parkinson’s drugs. At present, the synthesis methods of acetylpyrazine include oxidation method, multi-step method and Grignard reagent method, which have the disadvantages of low yield, cumbersome process, severe reaction conditions and high cost. In this study, acetylation of pyrazine was used to synthesize acetylpyrazine by electrochemical method for the first time. In this reaction, ammonium persulfate was electrolyzed on lead electrode to generate sulfate radicals, which react with pyruvic acid to generate acetyl groups, and followed by reacting with protonated pyrazines to synthesize acetylpyrazines under acidic conditions. Firstly, the effects of various electrolysis conditions on the yield of acetylpyrazine were investigated, and the optimal electrolysis conditions were obtained. A volume ration of 1:1 between ammonium persulfate saturated aqueous solution and methylene chloride solution containing 1 mol·L-1 of pyrazine and 0.33 mol·L-1 of pyruvic acid were used as the catholyte. A lead plate was used as the cathode. The electrolysis was carried out at the current density of 100 A·m-2 under normal temperature and pressure. When the charge was 2.5 F·mol-1, the yield of acetylpyrazine reached 44.12%. In addition, iron electrodes and added ferrous sulfate were used to investigate the influence of electrochemical-transition metal composite activation method on the yield of acetylpyrazine. However, the composite activation method has little effect on the improvement of the yield of acetylpyrazine. In general, the electrochemical synthesis of acetylpyrazine is simple and easy to control. Moreover, the reaction is gentler, the product purity is high, thus, the separation steps are simplified, and the production cost is reduced. The use of “clean energy” electrons instead of transition metal salts as the reducing agent is an environmentally friendly preparation method with broad prospects. At the same time, ammonium sulfate can be oxidized at the anode to generate ammonium persulfate, while acetylpyrazine is synthesized at the cathode. Therefore, pyrazine acetylation is a direct and effective method for preparing acetylpyrazine and electrochemical synthesis of acetylpyrazine has broad industrial application prospects.

/
| 〈 |
|
〉 |