

调控Pt3Zn合金化程度改善酸性氧还原活性与稳定性
收稿日期: 2021-06-08
修回日期: 2021-07-11
网络出版日期: 2021-07-29
基金资助
科技部重点研发计划(2017YFA0206500);国家自然科学基金项目(21773198);国家自然科学基金项目(U1705253)
Adjusting the Alloying Degree of Pt3Zn to Improve Acid Oxygen Reduction Activity and Stability
Received date: 2021-06-08
Revised date: 2021-07-11
Online published: 2021-07-29
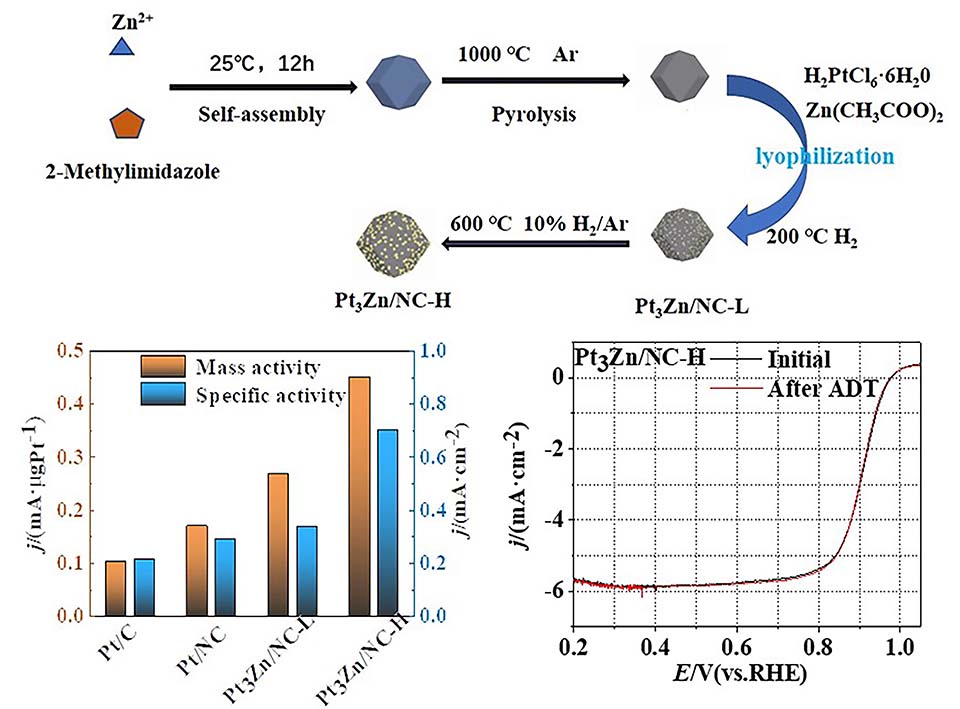
Pt基催化剂是氧还原反应的优良催化剂,改善其活性和稳定性是燃料电池商业化的关键。本文利用金属有机框架衍生的氮掺杂碳材料为载体,通过浸渍、冻干和简单热处理的方法合成了Pt/NC、Pt3Zn/NC-L和Pt3Zn/NC-H催化剂,平均粒径均在2 nm左右。在Pt中引入Zn元素引起其晶格收缩,使Pt-Pt键变短,优化了Pt与含氧中间体的结合,增强氧还原反应的活性。在高合金化程度的Pt3Zn/NC-H催化剂上,氧还原反应的半波电位为0.903 V,较商业Pt/C正移57 mV。0.9 V下的质量活性和面积比活性分别是商业Pt/C的4.50倍和3.33倍。在O2饱和的0.1 mol·L-1 HClO4溶液,0.6 ~ 1.0 V(vs. RHE)进行10000周稳定性测试,商业Pt/C的质量活性和面积比活性分别衰减25.00%和23.80%,而在Pt3Zn/NC-H催化剂上未观察到衰减。
张天恩 , 颜雅妮 , 张俊明 , 瞿希铭 , 黎燕荣 , 姜艳霞 . 调控Pt3Zn合金化程度改善酸性氧还原活性与稳定性[J]. 电化学, 2022 , 28(4) : 2106091 . DOI: 10.13208/j.electrochem.210609
Proton exchange membrane fuel cell (PEMFC) is a new type of energy device, a relatively excellent way to achieve carbon neutrality. However, due to the relatively slow reaction rate of oxygen reduction reaction (ORR) at the cathode, platinum (Pt) is the key material of the cathode catalyst. However, Pt is a kind of noble metal, and its high cost restricts the PEMFC commercialization process. At present, the main approach is to combine transition metals with Pt to prepare Pt-based alloys and to reduce the use of Pt. Pt-based alloys are excellent catalysts for ORR, improving both the activity and stability, and increasing the Pt utilization rate and the number of active sites of the catalyst. In this paper, by employing nitrogen-doped carbon material derived from a metal organic framework as a support, Pt/NC, Pt3Zn/NC-L and Pt3Zn/NC-H catalysts were successfully synthesized by impregnation, freeze-drying and simple heat treatment. The particle sizes were around 2 nm and uniformly supported on the carbon. Raman data shows that the defect degree was slightly reduced after loading metal, mainly because the nanoparticles would be anchored in the defect position, and the metal would help the graphitization of carbon at high temperature. The introduction of Zn into Pt caused the Pt lattice to be shrunk, which shortens the Pt-Pt bond length, and optimizes the combination of Pt and oxygen-containing intermediates, ultimately enhances the ORR activity. On the highly alloyed Pt3Zn/NC-H catalyst, the half-wave potential was 0.903 V, which is a positive shift of 57 mV compared with commercial Pt/C, moreover, the mass activity and area specific activity at 0.9 V were 4.50 times and 3.33 times to those of commercial Pt/C, respectively. The 10000-cycle stability test was carried out in an O2-saturated 0.1 mol·L-1 HClO4 solution at 0.6 ~ 1.0 V (vs. RHE). The mass activity and area specific activity of commercial Pt/C decreased by 25.00% and 23.80%, respectively, while Pt3Zn/NC-H catalyst revealed excellent stability. Transmission electron microscopic (TEM) observation shows that after the stability test, the nanoparticles were well dispersed on the nitrogen-doped carbon support, however, the commercial Pt/C became a slight agglomeration. Small particle sized Pt-based catalysts, constructed with a metal-organic framework-derived nitrogen-doped carbon material as a carrier, can improve the electrocatalytic activity and stability toward ORR, providing new ideas for the design and construction of Pt-based oxygen reduction catalysts.

| [1] | Wang T Y, Liang J S, Zhao Z L, Li S Z, Lu G, Xia Z C, Wang C, Luo J H, Han J T, Ma C, Huang Y, Li Q. Sub-6 nm fully ordered L10-Pt-Ni-Co nanoparticles enhance oxygen reduction via Co doping induced ferromagnetism enhancement and optimized surface strain[J]. Adv. Energy Mater., 2019, 9(17): 1803771. |
| [2] | Colón-Mercado H R, Kim H, Popov B N. Durability study of Pt3Ni1 catalysts as cathode in PEM fuel cells[J]. Electrochem Commun., 2004, 6(8): 795-799. |
| [3] | Yoo T Y, Yoo J M, Sinha A K, Bootharaju M S, Jung E, Lee H S, Lee B H, Kim J, Antink W H, Kim Y M, Lee J, Lee E, Lee D W, Cho S P, Yoo S J, Sung Y E, Hyeon T. Direct synthesis of intermetallic platinum-alloy nanoparticles highly loaded on carbon supports for efficient electrocatalysis[J]. J. Am. Chem. Soc., 2020, 142(33): 14190-14200. |
| [4] | Xiong Y, Xiao L, Yang Y, DiSalvo F J, Abruña H D. High-loading intermetallic Pt3Co/C core-shell nanoparticles as enhanced activity electrocatalysts toward the oxygen reduction reaction (ORR)[J]. Chem Mater., 2018, 30(5): 1532-1539. |
| [5] | Tian X L, Zhao X, Su Y Q, Wang L J, Wang H M, Dang D, Chi B, Liu H F, Hensen E J M, Lou X W, Xia B Y. Engineering bunched Pt-Ni alloy nanocages for efficient oxygen reduction in practical fuel cells[J]. Science, 2019, 366(6467): 850-856. |
| [6] | Escudero-Escribano M, Malacrida P, Hansen M H, Vej-Hansen U G, Velazquez-Palenzuela A, Tripkovic V, Schiotz J, Rossmeisl J, Stephens I E L, Chorkendorff I. Tuning the activity of Pt alloy electrocatalysts by means of the lanthanide contraction[J]. Science, 2016, 352(6281): 73-76. |
| [7] | Glüsen A, Dionigi F, Paciok P, Heggen M, Müller M, Gan L, Strasser P, Dunin-Borkowski R E, Stolten D. Dealloyed PtNi-core-shell nanocatalysts enable significant lowering of Pt electrode content in direct methanol fuel cells[J]. ACS Catal., 2019, 9(5): 3764-3772. |
| [8] | Wu D F, Zhang W, Lin A J, Cheng D J. Low Pt-content ternary PtNiCu nanoparticles with hollow interiors and accessible surfaces as enhanced multifunctional electrocatalysts[J]. ACS Appl. Mater. Interfaces, 2020, 12(8): 9600-9608. |
| [9] | Zhang B T, Fu G T, Li Y T, Liang L C, Grundish N S, Tang Y W, Goodenough J B, Cui Z M. General strategy for synthesis of ordered Pt3M intermetallics with ultrasmall particle size[J]. Angew. Chem. Int. Ed., 2020, 59(20): 7857-7863. |
| [10] | Liang J S, Zhao Z L, Li N, Wang X M, Li S Z, Liu X, Wang T Y, Lu G, Wang D L, Hwang B J, Huang Y H, Su D, Li Q. Biaxial strains mediated oxygen reduction electrocatalysis on fenton reaction resistant L10-PtZn fuel cell cathode[J]. Adv. Energy Mater., 2020, 10(29): 2000179. |
| [11] | Morozan A, Jousselme B, Palacin S. Low-platinum and platinum-free catalysts for the oxygen reduction reaction at fuel cell cathodes[J]. Energy Environ. Sci., 2011, 4(4): 1238-1254. |
| [12] | Seh Z W, Kibsgaard J, Dickens C F, Chorkendorff I B, Norskov J K, Jaramillo T F. Combining theory and experiment in electrocatalysis: Insights into materials design[J]. Science, 2017, 355(6321): eaad4998. |
| [13] | Xue Y K, Li H Q, Ye X W Y, Yang S L, Zheng Z P, Han X, Zhang X B, Chen L N, Xie Z X, Kuang Q, Zheng L S. N-doped carbon shell encapsulated PtZn intermetallic nanoparticles as highly efficient catalysts for fuel cells[J]. Nano Res., 2019, 12(10): 2490-2497. |
| [14] | Kim J, Rong C B, Lee Y, Liu J P, Sun S H. From core/shell structured FePt/Fe3S4/MgO to ferromagnetic FePt nanoparticles[J]. Chem. Mater., 2008, 20(23): 7242-7245. |
| [15] | Qi Z Y, Xiao C X, Liu C, Goh T W, Zhou L, Maligal-Ganesh R, Pei Y C, Li X L, Curtiss L A, Huang W Y. Sub-4 nm PtZn intermetallic nanoparticles for enhanced mass and specific activities in catalytic electrooxidation reaction[J]. J. Am. Chem. Soc., 2017, 139(13): 4762-4768. |
| [16] | Li J Z, Chen M J, Cullen D A, Hwang S, Wang M Y, Li B Y, Liu K X, Karakalos S, Lucero M, Zhang H G, Lei C, Xu H, Sterbinsky G E, Feng Z X, Su D, More K L, Wang G F, Wang Z B, Wu G. Atomically dispersed manganese catalysts for oxygen reduction in proton-exchange membrane fuel cells[J]. Nature Catalysis, 2018, 1(12): 935-945. |
| [17] | Wang J, Huang Z Q, Liu W, Chang C R, Tang H L, Li Z J, Chen W X, Jia C J, Yao T, Wei S Q, Wu Y E, Li Y D. Design of N-coordinated dual-metal sites: A stable and active Pt-free catalyst for acidic oxygen reduction reaction[J]. J. Am. Chem. Soc., 2017, 139(48): 17281-17284. |
| [18] | Zhang Z C, Tian X C, Zhang B W, Huang L, Zhu F C, Qu X M, Liu L, Liu S, Jiang Y X, Sun S G. Engineering phase and surface composition of Pt3Co nanocatalysts: A strategy for enhancing CO tolerance[J]. Nano Energy, 2017, 34: 224-232. |
| [19] | Zhao W Y, Ye Y K, Jiang W J, Li J, Tang H B, Hu J S, Du L, Cui Z M, Liao S J. Mesoporous carbon confined intermetallic nanoparticles as highly durable electrocatalysts for the oxygen reduction reaction[J]. J. Mater. Chem. A, 2020, 8(31): 15822-15828. |
| [20] | Ao X, Zhang W, Zhao B, Ding Y, Nam G, Soule L, Abdelhafiz A, Wang C D, Liu M L. Atomically dispersed Fe-N-C decorated with Pt-alloy core-shell nanoparticles for improved activity and durability towards oxygen reduction[J]. Energy Environ. Sci., 2020, 13(9): 3032-3040. |
| [21] | Hu Q P(胡清平), Tao Z Y(陶芝勇), Xiao L(肖丽), Zhuang L(庄林), Lu J T(陆君涛), Li X H(李新海). Study on preparation and electrochemical performance of the PdNi/C catalysts[J]. Chem. World(化学世界), 2018, 59(6):376-380. |
| [22] | Tayal J, Rawat B, Basu S. Bi-metallic and tri-metallic Pt-Sn/C, Pt-Ir/C, Pt-Ir-Sn/C catalysts for electro-oxidation of ethanol in direct ethanol fuel cell[J]. Int. J. Hydrogen Energ., 2011, 36(22): 14884-14897. |
| [23] | Zhu J, Zheng X, Wang J, Wu Z X, Han L L, Lin R Q, Xin H L L, Wang D L. Structurally ordered Pt-Zn/C series nano-particles as efficient anode catalysts for formic acid electrooxidation[J]. J. Mater. Chem. A, 2015, 3(44): 22129-22135. |
| [24] | Li J R, Sharma S, Wei K C, Chen Z T, Morris D, Lin H H, Zeng C, Chi M F, Yin Z Y, Muzzio M, Shen M Q, Zhang P, Peterson A A, Sun S H. Anisotropic strain tuning of L10 ternary nanoparticles for oxygen reduction[J]. J. Am. Chem. Soc., 2020, 142(45): 19209-19216. |
| [25] | Sheng Z H, Shao Lin, Chen J J, Bao W J, Wang F B, Xia X H. Catalyst-free synthesis of nitrogen-doped graphene via thermal annealing graphite oxide with melamine and its excellent electrocatalysis[J]. ACS Nano, 2011, 5(6): 4350-4358. |
| [26] | Zhang J W, Yuan Y L, Gao L, Zeng G M, Li M F, Huang H W. Stabilizing Pt-based electrocatalysts for oxygen reduction reaction: Fundamental understanding and design strategies[J]. Adv. Mater., 2021, 33(20): 2006494. |
| [27] | Liu J, Bak J, Roh J, Lee K S, Cho A, Han J W, Cho E. Reconstructing the coordination environment of platinum single-atom active sites for boosting oxygen reduction reaction[J]. ACS Catal., 2021, 11(1): 466-475. |
/
| 〈 |
|
〉 |