

ORR催化剂Nim@Pt1Aun-m-1 (n = 19, 38, 55, 79; m = 1, 6, 13, 19)的密度泛函研究
收稿日期: 2021-03-27
修回日期: 2021-05-08
网络出版日期: 2021-06-09
DFT Study of Nim@Pt1Aun-m-1 (n=19, 38, 55, 79; m = 1, 6, 13, 19) Core-Shell ORR Catalyst
Received date: 2021-03-27
Revised date: 2021-05-08
Online published: 2021-06-09
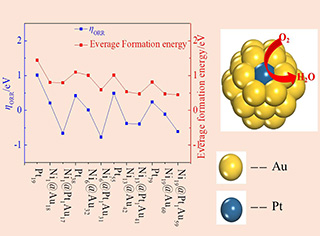
燃料电池的阴极反应的反应动力学速率非常慢,限制了燃料电池技术的发展。因此,寻找低成本、高活性的氧还原催化剂具有重要的意义。多元金属核壳团簇表现出优良的氧还原活性。在本文中,以原子个数为19、38、55和79的八面体团簇作催化剂模型,采用密度泛函理论(GGA-PBE-PAW)方法,研究了一系列不同尺寸核壳Nim@Mn-m (n = 19, 38, 55, 79;m = 1, 6, 13, 19; M = Pt, Pd, Cu, Au, Ag)团簇催化剂的活性规律。优化*O、*OH和*OOH吸附中间体结构,计算了吸附自由能和反应吉布斯自由能,以超电势为催化活性的描述符,研究了单原子Pt嵌入Nim@Aun-m团簇的活性规律。结果表明,Ni6@Pt1Au31具有最好的ORR活性,并且Ni1@Pt1Au17、Ni6@Pt1Au31、Ni13@Pt1Au41、Ni19@Pt1Au5表现出比Pt38团簇以及Pt(111)表面更高的催化活性。Bader电荷和态密度分析表面,核壳之间的电荷转移以及单原子Pt嵌入Nim@Aun-m表面,改变了吸附位的电子性质,降低了*OH的吸附强度,提高了ORR活性。单原子Pt嵌入Nim@Aun-m表面可能是一种合适的多元金属核壳ORR催化剂设计策略。
李文杰 , 田东旭 , 杜红 , 燕希强 . ORR催化剂Nim@Pt1Aun-m-1 (n = 19, 38, 55, 79; m = 1, 6, 13, 19)的密度泛函研究[J]. 电化学, 2021 , 27(4) : 357 -365 . DOI: 10.13208/j.electrochem.210329
The slow kinetics of oxygen reduction reaction (ORR) limits the performance of low temperature fuel cells. Thus, it needs to design effective catalysts with low cost. Core-shell clusters (CSNCs) show promising activity because of their size-dependent geometric and electronic effects. The ORR activity trend of Nim@Pt1Aun-m-1(n = 19, 38, 55, 79; m = 1, 6, 13, 19) was studied using the GGA-PBE-PAW methods. The adsorption configurations of *O, *OH and *OOH were optimized and the reaction free energies of four proton electron (H+ + e-) transfer steps were calculated. Using overpotential as a descriptor for the catalytic activity, Ni6@Pt1Au31 was found to be the most active ORR catalyst. Ni1@Pt1Au17, Ni13@Pt1Au41, and Ni19@Pt1Au59 had better activity than pure Pt clusters and Pt(111). Bader charge and DOS data indicate that the single Pt atom embedded on Nim@Aun-m can tune the electronic property of active site, and thus, significantly improve the activity. The present study showed that the single Pt atom embedded on Nim@Aun-m is a rational strategy to design effective core-shell ORR catalysts.

| [1] | Antolini E, Passos R R, Ticianelli E A. Electrocatalysis of oxygen reduction on a carbon supported platinum-vanadium alloy in polymer electrolyte fuel cells[J]. Electrochim. Acta., 2002, 48(3): 263-270. |
| [2] | Gasteiger H A, Kocha S S, Sompalli B, Wagner F T. Activity benchmarks for Pt, Pt-alloy and non-Pt oxygen reduction catalysts for PEMFCs[J]. Appl. Catal. B - Environ., 2005, 56(1-2): 9-35. |
| [3] | Debe M K. Electrocatalyst approaches and challenges for automotive fuel cells[J]. Nature, 2012, 486(7401): 43-51. |
| [4] | Chen J, Lim B, Lee E P, Xia Y. Shape-controlled synjournal of platinum nanocrystals for catalytic and electrocatalytic applications[J]. Nano Today, 2009, 4(1): 81-95. |
| [5] | Guo S J, Wang E K. Noble metal nanomaterials: Controllable synjournal and application in fuel cells and analytical sensors[J]. Nano Today, 2011, 6(3): 240-264. |
| [6] | Koenigsmann C, Santulli A C, Gong K, Vukmirovic M B, Zhou W P, Sutter E, Wong S S, Adzic R R. Enhanced electrocatalytic performance of processed, ultrathin, supported Pd-Pt core-shell nanowire catalysts for the oxygen reduction reaction[J]. J. Am. Chem. Soc., 2011, 133(25): 9783-9795. |
| [7] | Koenigsmann C, Sutter E, Chiesa T A, Adzic R R, Wong S S. Highly enhanced electrocatalytic oxygen reduction performance observed in bimetallic palladium-based nano-wires prepared under ambient, surfactantless conditions[J]. Nano Lett., 2012, 12(4): 2013-2020. |
| [8] | Sun S H, Zhang G X, Geng D S, Chen Y G, Li R Y, Cai M, Sun X L. A highly durable platinum nanocatalyst for proton exchange membrane fuel cells: multiarmed starlike nanowire single crystal[J]. Angew. Chem. Int. Ed., 2010, 50(2): 422-426. |
| [9] | Yang P. Platinum-based electrocatalysts with core-shell nanostructures[J]. Angew. Chem. Int. Ed., 2011, 50(12): 2674-2676. |
| [10] | Strasser P, Koh S, Anniyev T, Greeley J, Nilsson A. Lattice-strain control of the activity in dealloyed core-shell fuel cell catalysts[J]. Nature Chem., 2010, 2(6): 454-460. |
| [11] | Guo S J, Zhang S, Sun S H. Tuning nanoparticle catalysis for the oxygen reduction reaction[J]. Angew. Chem. Int. Ed., 2013, 52(33): 8526-8544. |
| [12] | Mani P, Srivastava R, Strasser P. Dealloyed PtCu coreshell nanoparticle electrocatalysts for use in PEM fuel cell cathodes[J]. J. Phys. Chem. C, 2012, 112(7): 2770-2778. |
| [13] | Wang D L, Xin H L L, Hovden R, Wang H S, Yu Y C, Muller D A, Disalvo F J, Abrua H C. Structurally ordered intermetallic platinum-cobalt core-shell nanoparticles with enhanced activity and stability as oxygen reduction electrocatalysts[J]. Nat. Mater., 2012, 12(1): 81-87. |
| [14] | Wang G X, Wu H M, Wexler D, Liu H K, Savadogo O. Ni@Pt core-shell nanoparticles with enhanced catalytic activity for oxygen reduction reaction[J]. J. Alloy. Compd., 2010, 503(1): L1-L4. |
| [15] | Neergat M, Rahul R. Unsupported Cu-Pt core-shell nano-particles: Oxygen reduction reaction (ORR) catalyst with better activity and reduced precious metal content[J]. J. Electrochem. Soc., 2012, 159(7): F234-F241. |
| [16] | Jang J H, Lee E, Park J, Kim G, Hong S, Kwon Y U. Rational syntheses of core-shell Fex@Pt nanoparticles for the study of electrocatalytic oxygen reduction reaction[J]. Sci. Rep., 2013, 3: 2872. |
| [17] | Chen Y M, Liang Z X, Yang F, Liu Y W, Chen S L. Ni-Pt core-shell nanoparticles as oxygen reduction electrocatalysts: Effect of Pt shell coverage[J]. J. Phys. Chem. C, 2011, 115(49): 24073-24079. |
| [18] | Zhang Y F, Qin J, Leng D Y, Liu Q R, Xu X Y, Yang B, Yin F. Tunable strain drives the activity enhancement for oxygen reduction reaction on Pd@Pt core-shell electrocatalysts[J]. J. Power Sources, 2021, 485: 229340. |
| [19] | Park J, Zhang L, Choi S I, Roling L T, Lu N, Herron J A, Xie S F, Wang J G, Kim M J, Mavrikakis M, Xia Y N. Atomic layer-by-layer deposition of platinum on palladium octahedra for enhanced catalysts toward the oxygen reduction reaction[J]. ACS Nano, 2015, 9(3): 2635-2647. |
| [20] | Zhang L, Iyyamperumal R, Yancey D F, Crooks R M, Henkelman G. Design of Pt-shell nanoparticles with alloy cores for the oxygen reduction reaction[J]. ACS Nano, 2013, 7(10): 9168-9172. |
| [21] | Strasser P, Koh S, Anniyev T, Greeley J, More K, Yu C, Liu Z, Kaya S, Nordlund D, Ogasawara H, Toney M F, Nilsson A. Lattice-strain control of the activity in dealloyed core-shell fuel cell catalysts[J]. Nat. Chem., 2010, 2(6): 454-460. |
| [22] | Zhang J L, Vukmirovic M B, Sasaki K, Nilekar A U, Ma-vrikakis M, Adzic R R. Mixed-metal Pt monolayer electrocatalysts for enhanced oxygen reduction kinetics[J]. J. Am. Chem. Soc., 2005, 127(36): 12480-12481. |
| [23] | Nörskov J K, Rossmeisl J, Logadottir A, Lindqvist L, Kitchin J R, Bligaard T, Jónsson H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode[J]. J. Phys. Chem. B, 2004, 108(46): 17886-17892. |
| [24] | Cheng D J, Qiu X G, Yu H Y. Enhancing oxygen reduction reaction activity of Pt-shelled catalysts via subsurface alloying[J]. Phys. Chem. Chem. Phys., 2014, 16(38): 20377-20381. |
| [25] | Wang L L, Johnson D D. Predicted trends of core-shell preferences for 132 late transition-metal binary-alloy nanoparticles[J]. J. Am. Chem. Soc., 2009, 131(39): 14023-14029. |
| [26] | Shin J, Choi J H, Cha P R, Kim S K, Kim I, Lee S C, Jeong D S. Catalytic activity for oxygen reduction reaction on platinum-based core-shell nanoparticles: All-electron density functional theory[J]. Nanoscale, 2015, 7(38): 15830-15839. |
| [27] | Nair A, Pathak B. Computational screening for ORR activity of 3d transition metal based M@Pt core-shell clusters[J]. J. Phys. Chem. C, 2019, 127(6): 3634-3644. |
| [28] | Greeley J, Stephens I E L, Bondarenko A S, Johansson T P, Hansen H A, Jaramillo T F, Rossmeisl J, Chorkendorff I, Nørskov J K. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts[J]. Nat. Chem., 2009, 1(7): 552-556. |
| [29] | Bligaard T, Norskov J K, Dahl S, Matthiesen J, Christensen C H, Sehested J. The Brnsted-Evans-Polanyi relation and the volcano curve in heterogeneous catalysis[J]. J. Catal., 2004, 224(1): 206-217. |
| [30] | Sobrinho D G, Nomiyama R K, Chaves A S, Piotrowski M J, Silva J. Structure, electronic, and magnetic properties of binary PtnTM55-n (TM = Fe, Co, Ni, Cu, Zn) nano-clusters: A density functional theory investigation[J]. J. Phys. Chem. C, 2015, 119(27): 15669-15679. |
| [31] | Kresse G, Hafner J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium[J]. Phys. Rev. B, 1994, 49(20): 14251-14269. |
| [32] | Kresse G G, Furthmüller J J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set[J]. Phys. Rev. B, 1996, 54(16): 11169-11186. |
| [33] | Perdew J P, Yue W. Accurate and simple density functional for the electronic exchange energy: Generalized gradient approximation[J]. Phys. Rev. B, 1986, 33(12): 8800-8802. |
| [34] | Perdew J P, Chevary J A, Vosko S H, Jackson K A, Pederson M R, Singh D J, Fiolhais C. Erratum: Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation[J]. Phys. Rev. B, 1993, 46(11): 6671-6687. |
| [35] | Kresse G, Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method[J]. Phys. Rev. B, 1999, 59(3): 1758-1775. |
| [36] | Teter M P, Payne M C, Allan D C. Solution of Schröding-er’s equation for large systems[J]. Phys. Rev. B, 1989, 40(18): 12255-12263. |
| [37] | Grimme S. Accurate description of van der Waals complexes by density functional theory including empirical corrections[J]. J. Comput. Chem., 2004, 25(12): 1463-1473. |
| [38] | Lee K, Murray Amonn D, Kong L, Lundqvist B I, Langreth D C. A higher-accuracy van der waals density functional[J]. Phys. Rev. B, 2010, 82(8): 081101. |
| [39] | Henkelman G, Arnaldsson A, Jónsson H. A fast and robust algorithm for Bader decomposition of charge density[J]. Comput. Mater. Sci., 2006, 36(3): 354-360. |
| [40] | Grgur B N, Markovi N M, Ross P N. Temperature-dependent oxygen electrochemistry on platinum low-index single crystal surfaces in acid solutions[J]. Can. J. Chem., 1997, 75(11): 1465-1471. |
| [41] | Blizanac B B, Lucas C A, Gallagher M E, Arenz M, Ross P N, Markovic N M. Anion adsorption, CO oxidation, and oxygen reduction reaction on a Au(100) surface: The pH effect[J]. J. Phys. Chem. B, 2004, 108(2): 625-634. |
| [42] | Wang L, Zeng Z H, Ma C, Liu Y F, Giroux M, Chi M F, Jin J, Greeley J, Wang C. Plating precious metals on nonprecious metal nanoparticles for sustainable electrocatalysts[J]. Nano Lett., 2017, 17(6): 3391-3395. |
| [43] | Fang P P, Duan S, Lin X D, Anema J R, Li J F, Buriez O, Ding Y, Fan F R, Wu D Y, Ren B, Wang Z L, Amatore C, Tian Z Q. Tailoring Au-core Pd-shell Pt-cluster nanoparticles for enhanced electrocatalytic activity[J]. Chem. Sci., 2011, 2(3): 531-539. |
| [44] | Dinesh B, Jyh-Pin C, Che Y, Hu A, Yang Y T, Chen T Y. Programming ORR Activity of Ni/NiOx@Pd electrocatalysts via controlling depth of surface-decorated atomic Pt clusters[J]. ACS Omega, 2018, 3(8): 8733-8744. |
| [45] | Wang H, An W. Promoting the oxygen reduction reaction with gold at step/edge sites of Ni@AuPt core-shell nano-particles[J]. Catal. Sci. Technol., 2017, 7(3): 596-606. |
/
| 〈 |
|
〉 |